Abstract
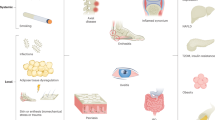
Psoriatic arthritis (PsA) is anatomically much more heterogeneous than rheumatoid arthritis, as, beyond synovitis, it often also involves enthesitis, peritendinitis, tenosynovitis, osteitis and periostitis. This heterogeneity currently precludes a gold standard for objectively defining resolution of inflammation following treatment, with enthesitis posing a particular challenge. Despite these difficulties, we apply lessons learned from rheumatoid arthritis to describe how patients with PsA and an inadequate response to therapy can be designated within two patient subgroups, characterized by persistent inflammatory PsA (PIPsA) and non-inflammatory PsA (NIPsA), respectively. The NIPsA phenotype is defined by the lack of ongoing joint inflammation, as confirmed through clinical assessment and imaging, along with normalized inflammatory marker levels. NIPsA might be associated with obesity, biomechanical-related pain, osteoarthritis, fibromyalgia, secondary post-inflammatory damage and central pain mechanisms. In this article, we frame PsA composite outcomes measures in relationship to the PIPsA and NIPsA phenotypes and propose that this approach might help to minimize unnecessary or ineffective cycling of PsA therapy in patients who acquire dominant non-inflammatory mechanisms and might also inform future trial design.
Key points
-
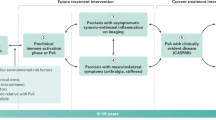
For optimal management of patients with psoriatic arthritis (PsA) and an inadequate response to treatment (particularly in cases that are difficult to treat or refractory), we propose two main disease subcategories: persistent inflammatory PsA (PIPsA) and non-inflammatory PsA (NIPsA).
-
Despite the complexity of PsA in terms of the structures involved (enthesis, synovium, tendons, para-tendinous soft tissue and bone), the best clinical feature for the routine recognition of genuine inflammatory arthritis is joint swelling (synovitis or dactylitis), which can be confirmed by ultrasonography (PIPsA phenotype).
-
In symptomatic patients with persistent pain and high composite scores, but without objective clinical signs of inflammation, the absence of ‘active’ inflammation on ultrasonography suggests a NIPsA phenotype that is likely to be associated with comorbidities, such as obesity and osteoarthritis; however, distinguishing ‘pure’ and less common isolated entheseal phenotypes remains challenging for this less common clinical phenotype.
-
The exhaustion of therapeutic options define treatment-refractory PsA; however, it is recognized that non-response, as measured by composite outcomes, might involve non-inflammatory components, highlighting the need for imaging.
-
Accurate characterization of the PIPsA phenotype will facilitate clinical trials, including combinations of advanced therapies using existing composite outcomes for PsA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gossec, L. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 83, 706–719 (2024).
Smolen, J. S. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 82, 3–18 (2023).
Marzo-Ortega, H. et al. Time to address the challenge of difficult to treat psoriatic arthritis: results from an international survey. Ann. Rheum. Dis. 83, 403–404 (2024).
Nagy, G. et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann. Rheum. Dis. 80, 31–35 (2021).
Perrotta, F. M., Scriffignano, S., Ciccia, F. & Lubrano, E. Clinical characteristics of potential ‘Difficult-to-treat’ patients with psoriatic arthritis: a retrospective analysis of a longitudinal cohort. Rheumatol. Ther. 9, 1193–1201 (2022).
Paudel, M. L. et al. Prevalence and characteristics of adults with difficult-to-treat rheumatoid arthritis in a large patient registry. Rheumatology https://doi.org/10.1093/rheumatology/keae318 (2024).
Winthrop, K. L. et al. Unmet need in rheumatology: reports from the advances in targeted therapies meeting, 2023. Ann. Rheum. Dis. 83, 409–416 (2024).
David, P. et al. Poly-refractory rheumatoid arthritis: an uncommon subset of difficult to treat disease with distinct inflammatory and noninflammatory phenotypes. Arthritis Rheumatol. 76, 510–521 (2024).
Singla, S., Ribeiro, A., Torgutalp, M., Mease, P. J. & Proft, F. Difficult-to-treat psoriatic arthritis (D2T PsA): a scoping literature review informing a GRAPPA research project. RMD Open. 10, e003809 (2024).
Lubrano, E., Scriffignano, S. & Perrotta, F. M. Difficult to treat and refractory to treatment in psoriatic arthritis. Rheumatol. Ther. 10, 1119–1125 (2023).
Glintborg, B. et al. Uptake and effectiveness of newer biologic and targeted synthetic disease-modifying antirheumatic drugs in psoriatic arthritis: results from five Nordic biologics registries. Ann. Rheum. Dis. 82, 820–828 (2023).
Hagège, B. et al. Remission and low disease activity in psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology 59, 1818–1825 (2020).
Gladman, D. D. et al. Residual disease activity in Canadian patients with psoriatic arthritis treated with advanced therapies: results from a multiregistry analysis (UNISON-PsA). J. Rheumatol. 51, 479–487 (2024).
Lubrano, E., Perrotta, F. M., Scriffignano, S., Coates, L. C. & Helliwell, P. Sustained very low disease activity and remission in psoriatic arthritis patients. Rheumatol. Ther. 6, 521–528 (2019).
Lubrano, E. et al. Assessment of the Patient Acceptable Symptom State (PASS) in psoriatic arthritis: association with disease activity and quality of life indices. RMD Open. 6, e001170 (2020).
S, S. et al. Psoriatic arthritis acceptable symptoms state: does sex make a difference? Rheumatol. Ther. 11, 1393–1402 (2024).
Buch, M. H., Eyre, S. & McGonagle, D. Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol. 17, 17–33 (2021).
Eshaghi, A. et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat. Commun. 12, 2078 (2021).
Kuhlmann, T. et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. 22, 78–88 (2023).
Schett, G. et al. Enthesitis: from pathophysiology to treatment. Nat. Rev. Rheumatol. 13, 731–741 (2017).
Gladman, D. D., Antoni, C., Mease, P., Clegg, D. O. & Nash, P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann. Rheum. Dis. 64, ii14–741 (2005). ii14-17.
Lindqvist, U. R. C. et al. The Swedish early psoriatic arthritis register — 2-year followup: a comparison with early rheumatoid arthritis. J. Rheumatol. 35, 668–673 (2008).
McGonagle, D., Lories, R. J. U., Tan, A. L. & Benjamin, M. The concept of a ‘synovio-entheseal complex’ and its implications for understanding joint inflammation and damage in psoriatic arthritis and beyond. Arthritis Rheum. 56, 2482–2491 (2007).
Aydin, S. Z., Bridgewood, C., Zabotti, A., Girolimetto, N. & McGonagle, D. The transition from enthesis physiological responses in health to aberrant responses that underpin spondyloarthritis mechanisms. Curr. Opin. Rheumatol. 33, 64–73 (2021).
McGonagle, D. G., Helliwell, P. & Veale, D. Enthesitis in psoriatic disease. Dermatol. Basel Switz. 225, 100–109 (2012).
Di Matteo, A. et al. Relationship Between ultrasound and physical examination in the assessment of enthesitis in patients with spondyloarthritis: results from the DEUS multicenter study. Arthritis Rheumatol. 77, 22–33 (2024).
Bakirci, S. et al. Entheseal changes in response to age, body mass index, and physical activity: an ultrasound study in healthy people. J. Rheumatol. 47, 968–972 (2020).
Michelsen, B. et al. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis. RMD Open. 3, e000486 (2017).
Kerschbaumer, A. et al. Efficacy of synthetic and biological DMARDs: a systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 82, 95–106 (2023).
Terslev, L. et al. Scoring ultrasound synovitis in rheumatoid arthritis: a EULAR-OMERACT ultrasound taskforce — part 2: reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open. 3, e000427 (2017).
Reddy, S. M. et al. Comparative analysis of disease activity measures, use of biologic agents, body mass index, radiographic features, and bone density in psoriatic arthritis and rheumatoid arthritis patients followed in a large U.S. disease registry. J. Rheumatol. 37, 2566–2572 (2010).
Merola, J. F., Chakravarty, S. D., Choi, O., Chan, D. & Gottlieb, A. B. A clinical review of structural damage in psoriatic arthritis for dermatologists: from pathogenesis to ongoing controversies. J. Am. Acad. Dermatol. 90, 349–357 (2024).
Honda, S. et al. Association of polygenic risk scores with radiographic progression in patients with rheumatoid arthritis. Arthritis Rheumatol. 74, 791–800 (2022).
Koc, G. H. et al. Determinants of radiographic progression in early psoriatic arthritis: insights from a real-world cohort. RMD Open. 10, e004080 (2024).
van der Heijde, D. et al. Radiographic progression according to baseline C-reactive protein levels and other risk factors in psoriatic arthritis treated with tofacitinib or adalimumab. J. Rheumatol. 46, 1089–1096 (2019).
Taylor, W. et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 54, 2665–2673 (2006).
Zabotti, A. et al. Risk of developing psoriatic arthritis in psoriasis cohorts with arthralgia: exploring the subclinical psoriatic arthritis stage. RMD Open. 10, e004314 (2024).
Mease, P. et al. Quantification of pre-existing radiographic damage and its relationship with joint activity and long-term clinical outcomes with secukinumab therapy in patients with psoriatic arthritis. Arthritis Res. Ther. 24, 283 (2022).
Dubash, S. et al. Dactylitis is an indicator of a more severe phenotype independently associated with greater SJC, CRP, ultrasound synovitis and erosive damage in DMARD-naive early psoriatic arthritis. Ann. Rheum. Dis. 81, 490–495 (2022).
Girolimetto, N. et al. Ultrasonographic evidence of predominance of acute extracapsular and chronic intrasynovial patterns in 100 cases of psoriatic hand dactylitis. J. Rheumatol. 47, 227–233 (2020).
Coates, L. C. et al. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat. Rev. Rheumatol. 18, 465–479 (2022).
Behrens, F. et al. Efficacy and safety of secukinumab in patients with spondyloarthritis and enthesitis at the Achilles tendon: results from a phase 3b trial. Rheumatology 61, 2856–2866 (2022).
Baraliakos, X. et al. Magnetic resonance imaging characteristics in patients with spondyloarthritis and clinical diagnosis of heel enthesitis: post hoc analysis from the phase 3 ACHILLES trial. Arthritis Res. Ther. 24, 111 (2022).
Zabotti, A. et al. Musculoskeletal ultrasonography for psoriatic arthritis and psoriasis patients: a systematic literature review. Rheumatology 56, 1518–1532 (2017).
Naredo, E. et al. Validation and incorporation of digital entheses into a preliminary GLobal OMERACT Ultrasound DActylitis Score (GLOUDAS) in psoriatic arthritis. Ann. Rheum. Dis. 83, 1060–1071 (2024).
Zabotti, A. et al. Novel and reliable DACTylitis glObal Sonographic (DACTOS) score in psoriatic arthritis. Ann. Rheum. Dis. 79, 1037–1043 (2020).
Di Matteo, A. et al. Power Doppler signal at the enthesis and bone erosions are the most discriminative OMERACT ultrasound lesions for SpA: results from the DEUS (Defining Enthesitis on Ultrasound in Spondyloarthritis) multicentre study. Ann. Rheum. Dis. 83, 847–857 (2024).
Gessl, I. et al. Tenderness and radiographic progression in rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 82, 344–350 (2023).
Smerilli, G. et al. Double target’ ultrasound monitoring of biologic therapy in psoriatic arthritis. Clin. Exp. Rheumatol. 42, 626–632 (2024).
Schett, G. et al. Psoriatic arthritis from a mechanistic perspective. Nat. Rev. Rheumatol. 18, 311–325 (2022).
Savage, L. et al. Defining pre-clinical psoriatic arthritis in an integrated dermato-rheumatology environment. J. Clin. Med. 9, 3262 (2020).
Castillo-Gallego, C., Aydin, S. Z., Emery, P., McGonagle, D. G. & Marzo-Ortega, H. Magnetic resonance imaging assessment of axial psoriatic arthritis: extent of disease relates to HLA-B27. Arthritis Rheum. 65, 2274–2278 (2013).
Girolimetto, N. et al. Sensitivity to change and clinical correlations of the novel DACtylitis glObal Sonographic (DACTOS) score in psoriatic arthritis. Rheumatology 60, 4103–4111 (2021).
Zabotti, A. et al. Ultrasonography in psoriatic arthritis: which sites should we scan? Ann. Rheum. Dis. 77, 1537–1538 (2018).
Aydin, S. Z. & Deodhar, A. Are all entheses the same? Rheumatology 63, 1–2 (2024).
Sakellariou, G. et al. Differential diagnosis of inflammatory arthropathies by musculoskeletal ultrasonography: a systematic literature review. Front. Med. 7, 141 (2020).
Gessl, I. et al. Systematic literature review to inform the EULAR recommendations for the use of imaging in crystal-induced arthropathies in clinical practice. Ann. Rheum. Dis. 83, 1208–1224 (2024).
Marchesoni, A. et al. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology 57, 32–40 (2018).
Marchesoni, A., De Lucia, O., Rotunno, L., De Marco, G. & Manara, M. Entheseal power Doppler ultrasonography: a comparison of psoriatic arthritis and fibromyalgia. J. Rheumatol. Suppl. 89, 29–31 (2012).
Zabotti, A., Mandl, P., Zampogna, G., Dejaco, C. & Iagnocco, A. One year in review 2018: ultrasonography in rheumatoid arthritis and psoriatic arthritis. Clin. Exp. Rheumatol. 36, 519–525 (2018).
Zabotti, A. et al. Enthesitis of the hands in psoriatic arthritis: an ultrasonographic perspective. Med. Ultrason. 19, 438–443 (2017).
Mandl, P. & Aletaha, D. The role of ultrasound and magnetic resonance imaging for treat to target in rheumatoid arthritis and psoriatic arthritis. Rheumatology 58, 2091–2098 (2019).
Dubash, S. R. et al. Ultrasound shows swollen joints are the better proxy for synovitis than tender joints in DMARD-naïve early psoriatic arthritis. Rheumatol. Adv. Pract. 5, rkab086 (2021).
Balint, P. V. et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative. Ann. Rheum. Dis. 77, 1730–1735 (2018).
Chandran, V., Liao, W. & de Vlam, K. Biomarkers in psoriasis and psoriatic arthritis: where are we now? J. Rheumatol. 51, 74–76 (2024).
Bowes, J. et al. Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat. Commun. 6, 6046 (2015).
Ritchlin, C. T. et al. Psoriatic arthritis subtypes are phenocopied in humanized mice. JCI Insight 9, e178213 (2024).
Kuiper, J. J. et al. EULAR study group on ‘MHC-I-opathy’: identifying disease-overarching mechanisms across disciplines and borders. Ann. Rheum. Dis. 82, 887–896 (2023).
O’Rielly, D. D., Jani, M., Rahman, P. & Elder, J. T. The genetics of psoriasis and psoriatic arthritis. J. Rheumatol. Suppl. 95, 46–50 (2019).
Merashli, M., De Marco, G., Podgorski, M., McGonagle, D. & Marzo-Ortega, H. Evidence of response to IL-6 inhibition in some cases of refractory spondyloarthritis-associated peripheral synovitis. Ann. Rheum. Dis. 75, 1418–1420 (2016).
Bridgewood, C. et al. T Helper 2 IL-4/IL-13 dual blockade with dupilumab is linked to some emergent T helper 17‒type diseases, including seronegative arthritis and enthesitis/enthesopathy, but not to humoral autoimmune diseases. J. Investig. Dermatol. 142, 2660–2667 (2022).
Ciliento, M. S. et al. Evaluation of the synovial effects of biological and targeted synthetic DMARDs in patients with psoriatic arthritis: a systematic literature review and meta-analysis. Int. J. Mol. Sci. 24, 5006 (2023).
Garaffoni, C. et al. High-grade synovitis associates with clinical markers and response to therapy in chronic inflammatory arthritis: post hoc analysis of a synovial biomarkers prospective cohort study. Front. Immunol. 14, 1298583 (2023).
Roseman, C. et al. Persistent pain and its predictors after starting anti-tumour necrosis factor therapy in psoriatic arthritis: what is the role of inflammation control? Scand. J. Rheumatol. 53, 94–103 (2024).
Samuel, C. et al. Characteristics associated with patient-reported treatment success in psoriatic arthritis. Rheumatology https://doi.org/10.1093/rheumatology/keae149 (2024).
Trouvin, A.-P. & Perrot, S. New concepts of pain. Best. Pract. Res. Clin. Rheumatol. 33, 101415 (2019).
Mease, P. J. Navigating the complexity of pain in psoriatic arthritis and axial spondyloarthritis. Curr. Opin. Rheumatol. 36, 282–288 (2024).
Horbal, N. & Maksymowych, W. P. Nociplastic pain in axial spondyloarthritis and psoriatic arthritis: role of JAK kinases in immunopathology and therapeutic impact of JAK inhibitors. Expert. Rev. Clin. Immunol. 21, 137–152 (2025).
Rifbjerg-Madsen, S. et al. Pain and pain mechanisms in patients with inflammatory arthritis: a Danish nationwide cross-sectional DANBIO registry survey. PLoS ONE 12, e0180014 (2017).
de Vlam, K. et al. Identifying and quantifying the role of inflammation in pain reduction for patients with psoriatic arthritis treated with tofacitinib: a mediation analysis. Rheumatol. Ther. 9, 1451–1464 (2022).
Sunzini, F., Schrepf, A., Clauw, D. J. & Basu, N. The biology of pain: through the rheumatology lens. Arthritis Rheumatol. 75, 650–660 (2023).
Aletaha, D., Alasti, F. & Smolen, J. S. Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann. Rheum. Dis. 76, 418–421 (2017).
Coates, L. C., Fransen, J. & Helliwell, P. S. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann. Rheum. Dis. 69, 48–53 (2010).
Leeb, B. F. et al. The disease activity score in 28 joints in rheumatoid arthritis and psoriatic arthritis patients. Arthritis Rheum. 57, 256–260 (2007).
Orbai, A.-M. et al. Updating the psoriatic arthritis (PsA) core domain set: a report from the PsA workshop at OMERACT 2016. J. Rheumatol. 44, 1522–1528 (2017).
Hjort, G., Schwarz, C. W., Skov, L. & Loft, N. Clinical characteristics associated with response to biologics in the treatment of psoriasis: a meta-analysis. JAMA Dermatol. 160, 830–837 (2024).
Gossec, L. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 79, 700–712 (2020).
Macchioni, P. et al. Ultrasonographic and clinical assessment of peripheral enthesitis in patients with psoriatic arthritis, psoriasis, and fibromyalgia syndrome: the ULISSE study. J. Rheumatol. 46, 904–911 (2019).
Mease, P., Reed, G., Ogdie, A., Pappas, D. A. & Kremer, J. M. Prevalence of fibromyalgia and widespread pain in psoriatic arthritis: association with disease severity assessment in a large US registry. Arthritis Care Res 76, 1313–1321 (2024).
Love, T. J. et al. Obesity and the risk of psoriatic arthritis: a population-based study. Ann. Rheum. Dis. 71, 1273–1277 (2012).
Green, A. et al. Modifiable risk factors and the development of psoriatic arthritis in people with psoriasis. Br. J. Dermatol. 182, 714–720 (2020).
Højgaard, P. et al. The influence of obesity on response to tumour necrosis factor-α inhibitors in psoriatic arthritis: results from the DANBIO and ICEBIO registries. Rheumatology 55, 2191–2199 (2016).
Di Matteo, A. et al. How normal is the enthesis by ultrasound in healthy subjects? Clin. Exp. Rheumatol. 38, 472–478 (2020).
Jacques, P. et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann. Rheum. Dis. 73, 437–445 (2014).
Felten, R., Duret, P.-M., Gottenberg, J.-E., Spielmann, L. & Messer, L. At the crossroads of gout and psoriatic arthritis: ‘psout’. Clin. Rheumatol. 39, 1405–1413 (2020).
Petsch, C. et al. Prevalence of monosodium urate deposits in a population of rheumatoid arthritis patients with hyperuricemia. Semin. Arthritis Rheum. 45, 663–668 (2016).
Krekeler, M., Baraliakos, X., Tsiami, S. & Braun, J. High prevalence of chondrocalcinosis and frequent comorbidity with calcium pyrophosphate deposition disease in patients with seronegative rheumatoid arthritis. RMD Open. 8, e002383 (2022).
Lubrano, E., Scriffignano, S. & Perrotta, F. M. Multimorbidity and comorbidity in psoriatic arthritis — a perspective. Expert. Rev. Clin. Immunol. 16, 963–972 (2020).
De Vicente Delmás, A. et al. Uveitis in psoriatic arthritis: study of 406 patients in a single university center and literature review. RMD Open. 9, e002781 (2023).
Zabotti, A. et al. The challenge of IBD-related arthritis screening questionnaires in early and predominantly entheseal phenotypes. Rheumatol. Ther. 11, 1321–1331 (2024).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Fobelo Lozano, M. J., Serrano Giménez, R. & Castro Fernández, M. Emergence of inflammatory bowel disease during treatment with secukinumab. J. Crohns Colitis 12, 1131–1133 (2018).
Ogdie, A. et al. Defining outcome measures for psoriatic arthritis: a report from the GRAPPA-OMERACT working group. J. Rheumatol. 44, 697–700 (2017).
Koduri, G. & Solomon, J. J. Identification, monitoring, and management of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 75, 2067–2077 (2023).
Bongartz, T. et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62, 1583–1591 (2010).
Radic, M., Martinovic Kaliterna, D. & Radic, J. Overview of vasculitis and vasculopathy in rheumatoid arthritis — something to think about. Clin. Rheumatol. 32, 937–942 (2013).
Ribeiro, A. L. et al. Deciphering difficult-to-treat psoriatic arthritis (D2T-PsA): a GRAPPA perspective from an international survey of healthcare professionals. Rheumatol. Adv. Pract. 8, rkae074 (2024).
McGonagle, D., Hermann, K.-G. A. & Tan, A. L. Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology 54, 29–38 (2015).
Tan, A. L., Grainger, A. J., Tanner, S. F., Emery, P. & McGonagle, D. A high-resolution magnetic resonance imaging study of distal interphalangeal joint arthropathy in psoriatic arthritis and osteoarthritis: are they the same? Arthritis Rheum. 54, 1328–1333 (2006).
Geenen, R. et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 77, 797–807 (2018).
Vela, J. et al. Quantitative sensory testing, psychological profiles and clinical pain in patients with psoriatic arthritis and hand osteoarthritis experiencing pain of at least moderate intensity. Eur. J. Pain 28, 310–321 (2024).
Tarannum, S. et al. Sex- and gender-related differences in psoriatic arthritis. Nat. Rev. Rheumatol. 18, 513–526 (2022).
Højgaard, P. et al. Gender differences in biologic treatment outcomes — a study of 1750 patients with psoriatic arthritis using Danish Health Care Registers. Rheumatology 57, 1651–1660 (2018).
Stober, C. et al. Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology 57, 158–163 (2018).
Eder, L., Thavaneswaran, A., Chandran, V. & Gladman, D. D. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann. Rheum. Dis. 72, 578–582 (2013).
Geijer, M. et al. The Swedish early psoriatic arthritis registry 5-year followup: substantial radiographic progression mainly in men with high disease activity and development of dactylitis. J. Rheumatol. 42, 2110–2117 (2015).
Kim, J.-R. & Kim, H. A. Molecular mechanisms of sex-related differences in arthritis and associated pain. Int. J. Mol. Sci. 21, 7938 (2020).
Luo, X. et al. IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice. Neuron 109, 2691–2706.e5 (2021).
Valero-Martínez, C. et al. Dual targeted therapy in patients with psoriatic arthritis and spondyloarthritis: a real-world multicenter experience from Spain. Front. Immunol. 14, 1283251 (2023).
Cuchacovich, R., Garcia-Valladares, I. & Espinoza, L. R. Combination biologic treatment of refractory psoriasis and psoriatic arthritis. J. Rheumatol. 39, 187–193 (2012).
Simon, D., Fagni, F. & Schett, G. Sequential interleukin-17/interleukin-23 inhibition in treatment-refractory psoriatic arthritis. Ann. Rheum. Dis. 81, 1334–1336 (2022).
Hren, M. G. & Khattri, S. Treatment of recalcitrant psoriasis and psoriatic arthritis with a combination of a biologic plus an oral JAK or TYK2 inhibitor: a case series. Ann. Rheum. Dis. 83, 1392–1393 (2024).
Feagan, B. G. et al. Guselkumab plus golimumab combination therapy versus guselkumab or golimumab monotherapy in patients with ulcerative colitis (VEGA): a randomised, double-blind, controlled, phase 2, proof-of-concept trial. Lancet Gastroenterol. Hepatol. 8, 307–320 (2023).
van Gestel, A. M., Haagsma, C. J. & van Riel, P. L. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 41, 1845–1850 (1998).
Nell-Duxneuner, V. P. et al. Evaluation of the appropriateness of composite disease activity measures for assessment of psoriatic arthritis. Ann. Rheum. Dis. 69, 546–549 (2010).
Helliwell, P. S. et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann. Rheum. Dis. 72, 986–991 (2013).
Prevoo, M. L. et al. Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatism Association preliminary remission criteria in relation to the disease activity score. Br. J. Rheumatol. 35, 1101–1105 (1996).
Fransen, J. et al. Performance of response criteria for assessing peripheral arthritis in patients with psoriatic arthritis: analysis of data from randomised controlled trials of two tumour necrosis factor inhibitors. Ann. Rheum. Dis. 65, 1373–1378 (2006).
Acknowledgements
D.M.’s work is supported by the Leeds National Institute of Health Research Biomedical Research Centre.
Author information
Authors and Affiliations
Contributions
A.Z., S.Z.A., A.D.M. and D.M. conceived the main concepts and equally described the newly suggested terminology. A.Z., S.Z.A., P.D., A.D.M. and D.M. contributed to writing. A.Z., A.D.M. and D.M. revised the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests to declare.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Alexis Ogdie, Rubén Queiro and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zabotti, A., Aydin, S.Z., David, P. et al. Delineating inflammatory from non-inflammatory mechanisms for therapy optimization in psoriatic arthritis. Nat Rev Rheumatol 21, 237–248 (2025). https://doi.org/10.1038/s41584-025-01229-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-025-01229-6