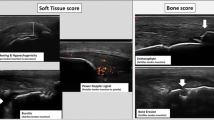
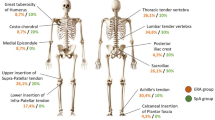
Abstract
Enthesitis-related arthritis (ERA), a distinct subtype of juvenile idiopathic arthritis (JIA) related to HLA-B27 and peripheral and axial involvement, presents with insidious onset of arthritis and/or enthesitis. However, there is a lack of data concerning axial new bone formation in patients transitioning into adulthood. To evaluate the axial radiographic structural damage (axRxSD), encompassing the sacroiliac joints (SIJ), hips, and spine, in ERA patients across various age groups. A cross-sectional cohort study was conducted with patients aged up to 35 years. Specific tools were used for measuring disease activity (BASDAI, ASDAS), function (BASFI, HAQ-S), mobility (BASMI), clinical enthesitis (MASES), ultrasound evaluation (MASEI), and axRxSD, including mSASSS for spine, Kellgren-Lawrence for hips and modified New York criteria for SIJ. A total of 26 patients were included, of whom 76.9% were males, with a mean age at diagnosis and assessment of 11.9 and 19.7 years, respectively. HLA-B27 positivity was found in 58.3%. Current active arthritis and enthesitis were present in 19.2% and 23%, respectively, with mean MASEI score of 12 (IQR 6–17). Peripheral joint limitation was observed in 50%, despite a BASMI score of 2.2 and 16% occurrence of abnormal FABER test. Most patients were in remission or low disease activity [ASDAS-ESR = 1.2 (0.6-2.3); ASDAS-CRP = 1.55 (0.6-2.4)]. Modified New York criteria were fulfilled by 73.1% of patients and 15.4% had radiographic hip involvement. Spine involvement, measured by mSASSS, was low (IQR 0-4.2), with only two patients exhibiting syndesmophytes. There was no statistical association between any imaging methods and clinical, laboratory, and ultrasound variables, including scores for activity, functionality, and mobility. Significant association was found only between axRxSD and BASMI. Our results showed high frequency of SIJ ankylosis alongside lower radiographic involvement in the spine and hips, suggesting a distinct structural damage phenotype. The early recognition of this outcome and the use of immunobiological therapy may mitigate syndesmophyte occurrence over time.
Graphical Abstract


Similar content being viewed by others
References
Petty RE, Southwood TR, Manners P et al (2004) International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31:390–392
Lovell DJ, Brunner HI (2021) Evolution in the understanding of Pediatric-Onset Axial Spondyloarthritis. Arthritis Care Res (Hoboken) 73:921–923. https://doi.org/10.1002/acr.24536
Burgos-Vargas R (2002) The juvenile-onset spondyloarthritides. Rheum Dis Clin North Am 28:531–vi. https://doi.org/10.1016/s0889-857x(02)00033-9
Stoll ML, Bhore R, Dempsey-Robertson M et al (2010) Spondyloarthritis in a pediatric population: risk factors for sacroiliitis. J Rheumatol 37:2402–2408. https://doi.org/10.3899/jrheum.100014
Sepriano A, Ramiro S, van der Heijde D et al (2020) What is axial spondyloarthritis? A latent class and transition analysis in the SPACE and DESIR cohorts. Ann Rheum Dis 79:324–331. https://doi.org/10.1136/annrheumdis-2019-216516
Martini A, Ravelli A, Avcin T et al (2019) Toward new classification criteria for juvenile idiopathic arthritis: first steps, Pediatric Rheumatology International trials Organization International Consensus. J Rheumatol 46:190–197. https://doi.org/10.3899/jrheum
Demir S, Ergen FB, Taydaş O et al (2022) Spinal involvement in juvenile idiopathic arthritis: what do we miss without imaging? Rheumatol Int 42:519–527. https://doi.org/10.1007/s00296-021-04890-8
Chan OM, Lai BM, Leung AS et al (2023) High prevalence of sacroiliitis and early structural changes in the sacroiliac joint in children with enthesitis-related arthritis: findings from a tertiary centre in Hong Kong. Pediatr Rheumatol Online J. https://doi.org/10.1186/s12969-023-00825-8
Naveen R, Mohindra N, Jain N et al (2021) Hip involvement in children with enthesitis related arthritis (ERA) is associated with poor outcomes in adulthood. Clin Rheumatol 40:4619–4627. https://doi.org/10.1007/s10067-021-05807-3
Rudwaleit M, Jurik AG, Hermann KG et al (2009) Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis 68:1520–1527. https://doi.org/10.1136/ard.2009.110767
Maksymowych WP, Lambert RG, Østergaard M et al (2019) MRI lesions in the sacroiliac joints of patients with spondyloarthritis: an update of definitions and validation by the ASAS MRI working group. Ann Rheum Dis 78:1550–1558. https://doi.org/10.1136/annrheumdis-2019-215589
Wang R, Bathon JM, Ward M (2020) Nonsteroidal antiinflammatory drugs as potential disease-modifying medications in Axial Spondyloarthritis. Arthritis Rheumatol 72:518–528. https://doi.org/10.1002/art.41164
Lee TH, Koo BS, Nam B et al (2022) Age-stratified trends in the progression of spinal radiographic damage in patients with ankylosing spondylitis: a longitudinal study. Ther Adv Musculoskelet Dis 23:14:1759720X221100301. https://doi.org/10.1177/1759720X221100301
Landewé R, Dougados M, Mielants H et al (2009) Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 68:863–867. https://doi.org/10.1136/ard.2008.091793
Oliveira TL, Silva FD, Filho AGO et al (2024) Relationship between spinal structural damage and sagittal balance in axial spondyloarthritis: is the thoracic spine the starting point? Semin Arthritis Rheum 65:152415. https://doi.org/10.1016/j.semarthrit.2024.152415
Baraliakos X, Østergaard M, Gensler LS et al (2020) SURPASS Study Group. Comparison of the effects of Secukinumab and Adalimumab Biosimilar on Radiographic Progression in patients with Ankylosing spondylitis: design of a Randomized, Phase IIIb Study (SURPASS). Clin Drug Investig 40:269–278. https://doi.org/10.1007/s40261-020-00886-7
Heuft-Dorenbosch L, Spoorenberg A, van Tubergen A et al (2003) Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 62:127–132. https://doi.org/10.1136/ard.62.2.127
Petty RE, Laxer RM, Lindsley CB et al (2021) Textbook of Pediatric Rheumatology. 8 th edition. Elsevier, Philadelphia
Berntson L, Damgård M, Andersson-Gäre B et al (2008) HLA-B27 predicts a more extended disease with increasing age at onset in boys with juvenile idiopathic arthritis. J Rheumatol 35:2055–2061
Macedo CSG, Souza PRA, Alves PM et al (2009) Study of validity and intra and inter-observer reliability of modified-modified Schöber test in subjects with low-back pain. Fisioter Pesqui 16:233–238
Jenkinson TR, Mallorie PA, Whitelock HC et al (1994) Defining spinal mobility in ankylosing spondylitis (AS). The bath AS Metrology Index. J Rheumatol 21:1694–1698
Garrett S, Jenkinson T, Kennedy LG et al (1994) A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing Spondylitis Disease Activity Index. J Rheumatol 21:2286–2291
Daltroy LH, Larson MG, Roberts NW et al (1990) A modification of the Health Assessment Questionnaire for the spondyloarthropathies. J Rheumatol 17:946–950
Calin A, Garrett S, Whitelock H et al (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing Spondylitis Functional Index. J Rheumatol 21:2281–2285
Lukas C, Landewé R, Sieper J et al (2009) Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis 68:18–24. https://doi.org/10.1136/ard.2008.094870
Machado P, Landewé R, Lie E et al (2011) Ankylosing Spondylitis Disease Activity score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 70:47–53. https://doi.org/10.1136/ard.2010.138594
Creemers MC, Franssen MJ, van’t Hof MA et al (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64:127–129. https://doi.org/10.1136/ard.2004.020503
van der Heide D, Braun J, Deodhar A et al (2019) Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford) 58:388–400. https://doi.org/10.1093/rheumatology/key128
van der Linden SM, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27:361–368. https://doi.org/10.1002/art.1780270401
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502. https://doi.org/10.1136/ard.16.4.494
De Miguel E, Cobo T, Muñoz-Fernández S et al (2009) Validity of enthesis ultrasound assessment in spondyloarthropathy. Ann Rheum Dis 68:169–174. https://doi.org/10.1136/ard.2007.084251
Falcao S, Castillo-Gallego C, Peiteado D et al (2015) Can we use enthesis ultrasound as an outcome measure of disease activity in spondyloarthritis? A study at the Achilles level. Rheumatology (Oxford) 54:1557–1562. https://doi.org/10.1093/rheumatology/keu399
Silva VB, Faquin G, Nicácio A et al (2013) Association between the ultrasonographic and clinical findings in the hips of patients with juvenile idiopathic arthritis. Rev Bras Reumatol 53:322–327
Sudoł-Szopińska I, Gietka P, Znajdek M et al (2017) Imaging of juvenile spondyloarthritis. Part I: classifications and radiographs. J Ultrason 17:167–175. https://doi.org/10.15557/JoU.2017.0025
Guła Z, Barczyńska T, Brzosko M et al (2017) Results from Polish Spondyloarthritis Initiative registry (PolSPI) - methodology and data from - the first year of observation. Reumatologia 55:59–64. https://doi.org/10.5114/reum.2017.67599
Watad A, Bridgewood C, Russell T et al (2018) The early phases of Ankylosing spondylitis: emerging insights from Clinical and Basic Science. Front Immunol 9:2668. https://doi.org/10.3389/fimmu.2018.02668
Benjamin M, Toumi H, Ralphs JR et al (2006) Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 208:471–490. https://doi.org/10.1111/j.1469-7580.2006.00540.x
Lassoued Ferjani H, Maatallah K, Miri S et al (2022) Enthesitis-related arthritis: monitoring and specific tools. J Pediatr (Rio J) 98:223–229. https://doi.org/10.1016/j.jped.2021.08.002
Akca UK, Batu ED, Sener S et al (2022) The performances of the ILAR, ASAS, and PRINTO classification criteria in ERA patients: a comparison study. Clin Rheumatol 41:1785–1792. https://doi.org/10.1007/s10067-022-06080-8
Skare TL, Leite N, Bortoluzzo AB et al (2012) Effect of age at disease onset in the clinical profile of spondyloarthritis: a study of 1424 Brazilian patients. Clin Exp Rheumatol 30:351–357
Skare TL, Bortoluzzo AB, Gonçalves CR et al (2012) Ethnic influence in clinical and functional measures of Brazilian patients with spondyloarthritis. J Rheumatol 39:141–147. https://doi.org/10.3899/jrheum.110372
Dos Reis Annunciato D, Oliveira TL, Magalhães VO et al (2023) Extra-musculoskeletal manifestations driving the therapeutic decision-making in patients with Spondyloarthritis: a 12-month follow-up prospective cohort study. Adv Rheumatol 63:44. https://doi.org/10.1186/s42358-023-00324-0
Lanças SHS, Furlan MZB, Fernandes TAP et al (2024) Presentation of enthesitis-related arthritis and juvenile-onset spondyloarthritis: a cross-sectional study in a pediatric and adult clinic. Adv Rheumatol 64:39. https://doi.org/10.1186/s42358-024-00378-8
Sari I, Lee S, Tomlinson G et al (2021) Factors Predictive of Radiographic Progression in Ankylosing Spondylitis. Arthritis Care Res (Hoboken) 73:275–281. https://doi.org/10.1002/acr.24104
Poddubnyy D, Haibel H, Listing J et al (2012) Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum 64:1388–1398. https://doi.org/10.1002/art.33465
Weiss PF, Xiao R, Brandon TG et al (2018) Radiographs in screening for sacroiliitis in children: what is the value? Arthritis Res Ther 20:141. https://doi.org/10.1186/s13075-018-1642-8
D’Agostino MA, Said-Nahal R, Hacquard-Bouder C et al (2023) Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power doppler: a cross-sectional study. Arthritis Rheum 48:523–533. https://doi.org/10.1002/art.10812
Rocha FAC, Pinto ACMD, Lopes JR, Deodhar A (2021) Tumor necrosis factor inhibitors prevent structural damage in hips in ankylosing spondylitis-time to reconsider treatment guidelines? A case series and review of literature. Clin Rheumatol 40:1881–1887. https://doi.org/10.1007/s10067-020-05519-0
Vander Cruyssen B, Vastesaeger N, Collantes-Estévez E (2013) Hip disease in ankylosing spondylitis. Curr Opin Rheumatol 25:448–454. https://doi.org/10.1097/BOR.0b013e3283620e04
Flatø B, Hoffmann-Vold AM, Reiff A et al (2006) Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum 54:3573–3582. https://doi.org/10.1002/art.22181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors don’t have financial or non-financial interests that are directly or indirectly related to the work submitted for publication. The authors state that they have full control of all primary data and agree to allow the journal to review their data if requested.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Araújo Pereira, A., do Amaral e Castro, A., Ahn, I. et al. Axial radiographic structural damage in patients with Enthesitis-Related Arthritis presents a distinct phenotype compared to adults with axial spondyloarthritis: A cross-sectional cohort study. Rheumatol Int 45, 54 (2025). https://doi.org/10.1007/s00296-025-05799-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00296-025-05799-2