Treatment Options for Autosomal Dominant Polycystic Kidney Disease (ADPKD): What Works and What Does Not
Alissar El Chediak @alissar_chediak
Dr. Alissar El Chediak is currently a transplant nephrology fellow at Vanderbilt University Medical Center. She was born and raised in Lebanon where she attended medical school and completed her internal medicine residency at the American University of Beirut, before pursuing her nephrology fellowship at Vanderbilt. Her research interests include increasing organ access to minorities, ABO-incompatible kidney transplant, treatment of COVID in kidney transplant patients, and simultaneous kidney-and-pancreas transplant.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disorder. However, there is still a scarcity in treatments that prevent or slow the progression to end stage kidney disease (ESKD). Throughout this blog post, we will revisit some of the available therapeutic medical and non-medical options that have been trialed in ADPKD.
Before delving into specific therapies for managing ADPKD, we should understand the pathways leading to cyst formation and enlargement. These pathways are the target for the different proposed treatments. It all starts with mutations of either the PKD1 gene encoding the Polycystin 1 Complex (PC1) on chromosome 16 or by mutations of the PKD2 gene encoding the Polycystin 2 Complex (PC2). The loss of PC1 and/or PC2 activity leads to measurable biochemical changes in several cellular signaling pathways, including elevated cyclic adenosine 3’,5’-monophosphate (cAMP) levels and increased activation of mechanistic target of rapamycin (mTOR) complex 1, extracellular signal-regulated kinase (ERK), and Janus kinase–signal transducer and activator of transcription (JAK–STAT) signaling pathways with reductions in intracellular calcium levels and 5ʹ-AMP-activated protein kinase (AMPK) activation. Subsequently, cyst formation, cellular proliferation, and alterations in the level of arginine vasopressin (AVP) as well as other metabolic dysregulation can occur.
Several therapies have emerged to treat ADPKD including vasopressin V2 receptor antagonist (Tolvaptan), mTOR inhibitors (Sirolimus, Everolimus), somatostatin analogue (Octreotide), glucoceramide synthase inhibitor (Venglustat), and much more. However, not all of these treatments have been successful in preventing progression of the disease.
Tolvaptan, a vasopressin receptor antagonist, is the only medication approved to slow kidney function decline in ADPKD patients at risk for rapid progression. As a reminder, vasopressin triggers a cascade of intracellular events that result in cystogenesis. Blocking vasopressin results in lower cyst burden and preserved kidney function. Two landmark trials supporting its use are the TEMPO and REPRISE trials.The TEMPO trial, which was the larger of the two, provided us with data that blockade of vasopressin V2 receptor inhibits cyst growth, thereby delaying additional adverse clinical outcomes. REPRISE trial results also confirmed that tolvaptan slowed disease progression in ADPKD.
Other drugs that have been trialed in ADPKD include mTOR inhibitors, metformin, somatostatin analogues and most recently Venglustat.
The mTOR pathway is central to cyst formation, so mTOR inhibitors were thought to be good options for treatment of ADPKD. Unfortunately, clinical trials failed to show any clinical evidence for their use. While Sirolimus can slow cyst growth, large randomized trials showed no benefit on kidney function or polycystic kidney growth in patients with ADPKD. Similarly, a two-year randomized clinical trial by Walz et al showed that Everolimus slowed the increase in total kidney volume of patients with ADPKD but did not slow the progression of renal impairment.
Metformin is another drug that can potentially slow eGFR decline. Brosnahan et al conducted a feasibility study which tested the safety and tolerability of metformin in ADPKD patients with mildly reduced kidney function. Secondary and exploratory outcomes were the effect of metformin compared with placebo on (1) the percentage change in total kidney volume (TKV) referenced to height (htTKV in mL/m) and (2) the change in eGFR over a 12-month period. At 12 months, eGFR decline was -0.41 vs. 3.35 mL/min/1.73 m2 in metformin vs. placebo groups, respectively. However, the difference was not statistically different. Kidney volume changes were similar in both groups.
Somatostatin and its analogs (octreotide and lanreotide) may reduce kidney and liver cyst fluid accumulation among patients with PKD. However, these agents have not been shown to slow the progression of kidney function decline and produce a variety of undesirable adverse effects. Therefore, these agents aren’t recommended for use in the treatment of ADPKD.
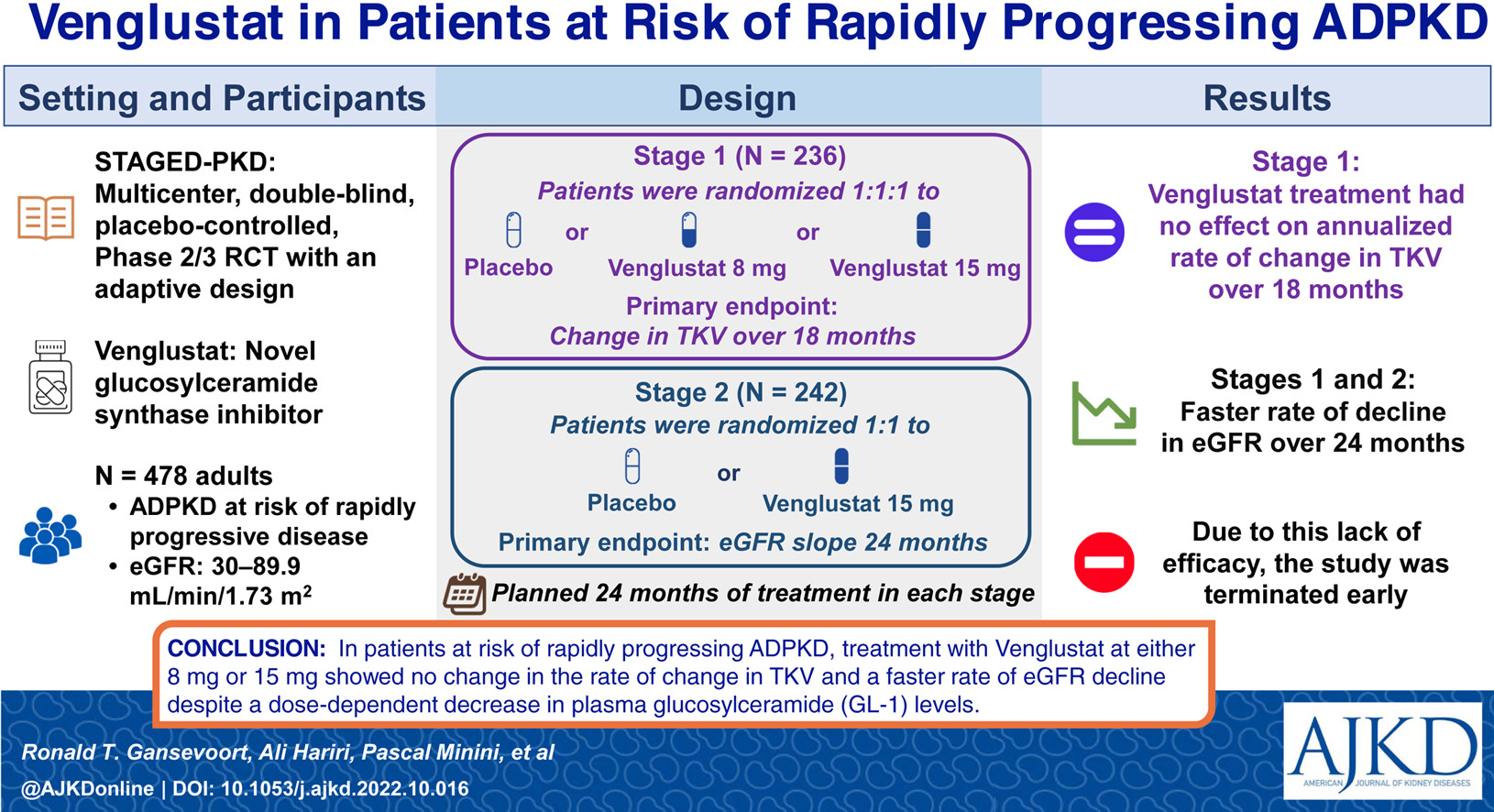
Alterations of glycosphingolipid metabolism and elevated glucosylceramide abundance have been documented in ADPKD. Low kidney glucosylceramide due to glucosylceramide synthase inhibitor resulted in effective inhibition of cystogenesis and fibrogenesis. Venglustat is a glucosylceramide synthase inhibitor that has been shown to inhibit cyst growth and reduce kidney failure in preclinical models of ADPKD. STAGED-PKD was a two-stage, multicenter, double-blind, randomized, placebo-controlled phase 2/3 study in adults with ADPKD at risk of rapidly progressive disease. In addition to the primary endpoints below, eGFR slope over 18 months (Stage 1), rate of change in TKV (Stage 2), safety/tolerability, pain, and fatigue (Stages 1 and 2) served as secondary endpoints. Venglustat treatment had no effect on the annualized rate of change in TKV over 18 months (Stage 1) and in fact had a faster rate of decline in eGFR slope over 24 months (Stage 2).
Several other drugs are currently under investigation to determine their efficacy in ADPKD patients. Besides pharmacotherapy, various surgical and interventional management options for ADPKD exist as well. Novel treatment modalities such as celiac plexus blockade and renal denervation appear to be promising in pain relief; however, further studies are needed to confirm their efficacy. Renal cyst decortication seems to have a higher success rate in targeting cyst-related pain compared with aspiration only. In terms of surgical interventions, some patients may benefit from native nephrectomy before or after a kidney transplant. Patients who are not candidates for native nephrectomy may consider transcatheter arterial embolization.
Major research efforts in both the clinical and preclinical setting in the last two decades resulted in a number of pivotal clinical trials aimed to address ADPKD. Several medications have been suggested to alter the course of kidney disease progression. However, the only medication that was proven effective in slowing kidney function decline has been Tolvaptan. mTOR inhibitors (Sirolimus, Everolimus), somatostatin analogue (Octreotide), and glucoceramide synthase inhibitor (Venglustat) have all been studied but proven to be non-effective. We anxiously await new therapies that can improve the outcomes of our patients with ADPKD.
– Post prepared by Alissar El Chediak @alissar_chediak
To view Gansevoort et al (Open Access), please visit AJKD.org.
Title: Venglustat, a Novel Glucosylceramide Synthase Inhibitor, in Patients at Risk of Rapidly Progressing ADPKD: Primary Results of a Double-Blind, Placebo-Controlled, Phase 2/3 Randomized Clinical Trial
Authors: Ronald T. Gansevoort, Ali Hariri, Pascal Minini, Curie Ahn, Arlene B. Chapman, Shigeo Horie, Bertrand Knebelmann, Michal Mrug, Albert C.M. Ong, York P.C. Pei, Vicente E. Torres, Vijay Modur, Igor Antonshchuk, and Ronald D. Perrone
DOI:10.1053/j.ajkd.2022.10.016

Leave a Reply