Abstract
Introduction/Methods
EPOCH-US is an ongoing, retrospective, observational cohort study among individuals identified in the Healthcare Integrated Research Database (HIRD®) with ≥ 12 months of continuous health plan enrollment. Data were collected for the HIRD population (containing immunocompetent and immunocompromised [IC] individuals), individual IC cohorts (non-mutually exclusive cohorts based on immunocompromising condition and/or immunosuppressive [IS] treatment), and the composite IC population (all unique IC individuals). This study updates previous results with addition of the general population cohort and data specifically for the year of 2022 (i.e., Omicron wave period). To provide healthcare decision-makers the most recent trends, this study reports incidence rates (IR) and severity of first SARS-CoV-2 infection; and relative risk, healthcare utilization, and costs related to first COVID-19 hospitalizations in the full year of 2022 and overall between April 2020 and December 2022.
Results
These updated results showed a 2.9% prevalence of immune compromise in the population. From April 2020 through December 2022, the overall IR of COVID-19 was 115.7 per 1000 patient-years in the composite IC cohort and 77.8 per 1000 patient-years in the HIRD cohort. The composite IC cohort had a 15.4% hospitalization rate with an average cost of $42,719 for first COVID-19 hospitalization. Comparatively, the HIRD cohort had a 3.7% hospitalization rate with an average cost of $28,848 for first COVID-19 hospitalization. Compared to the general population, IC individuals had 4.3 to 23 times greater risk of hospitalization with first diagnosis of COVID-19. Between January and December 2022, hospitalizations associated with first COVID-19 diagnosis cost over $1 billion, with IC individuals (~ 3% of the population) generating $310 million (31%) of these costs.
Conclusion
While only 2.9% of the population, IC individuals had a higher risk of COVID-19 hospitalization and incurred higher healthcare costs across variants. They also disproportionately accounted for over 30% of total costs for first COVID-19 hospitalization in 2022, amounting to ~ $310 million. These data highlight the need for additional preventive measures to decrease the risk of developing severe COVID-19 outcomes in vulnerable IC populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Despite reduced burden in the general population, immunocompromised populations continue to have a higher risk for severe COVID-19 outcomes, including prolonged hospitalization and death. |
What we learned from the study |
The current analysis updates data for the incidence of COVID-19 in immunocompromised populations from 1 April 2020 to 31 December 2022 in a commercially insured US population. Results add a general population cohort as well as hospitalizations, lengths of stay, and healthcare costs in the year 2022 (i.e., exclusively during the Omicron period) to provide healthcare decision-makers with the most relevant data and observed trends related to COVID-19 and SARS-CoV-2 infection in the immunocompromised population. |
The incidence rate of COVID-19 in the composite immunocompromised cohort between April 2020 and December 2022 was 115.7 cases per 1000 patient-years, compared to 77.8 per 1000 patient-years in the general population cohort. The chronic kidney disease stage 5/dialysis cohort consistently had the highest incidence rate (192.7 cases per 1000 patient-years overall). The difference in the incidence rates for the general population cohort and composite immunocompromised cohort was more pronounced during the later months of 2022. |
Despite immunocompromised individuals comprising less than 3% of the overall study population, their hospitalization expenses accounted for approximately 30% of the total costs incurred by individuals hospitalized for their initial COVID-19 diagnosis. Severe cases (39%) accounted for almost two-thirds of the $310 million in hospitalization expenses for COVID-19 in 2022. |
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused more than 767 million infections and over 6.9 million deaths globally since the start of the pandemic [1]. In the USA, more than 103 million cases, 6.2 million hospitalizations, and 1.1 million deaths have been attributed to COVID-19 [2, 3]. The total economic impact on the USA, including direct medical expenditures and approximated loss of revenue, is forecasted to reach $14 trillion by the end of 2023 [4, 5].
First reported in December 2019, COVID-19 initially had a mortality rate of 3.4% [6]. Since then, variants of the virus emerged rapidly, resulting in differing transmissibility levels, pathogenicity, and diminished vaccine effectiveness [6, 7]. In 2020, SARS-CoV-2 variants of concern included the Alpha (B.1.1.7) variant (UK, September 2020) and the Beta (B.1.351) variant (South Africa, May 2020) [6, 8, 9]. Gamma (Brazil, January 2021), Delta (India, June 2021), and Omicron (South Africa, November 2021) variants followed, with five major sub-lineages (BA.1. BA.2, BA.3, BA.4, BA.5) [6]. The evolution of variants has led to a more transmissible but seemingly less severe virus, and the number of deaths due to COVID-19 declined by 47% in the USA in 2022. Nonetheless, COVID-19 was the third leading cause of death in 2021 and the fourth leading cause of death in 2022 [10]. In May 2023, the World Health Organization (WHO) and the US Department of Health and Human Services declared an end to the COVID-19 public health emergency, resulting in changes to the type of public health data collected and insurance coverage for at-home COVID-19 test kits [11, 12].
As a result of the economic, social, and mental health hardships imposed by the virus and pandemic management strategies, public sentiment towards COVID-19 has also evolved alongside the emerging SARS-CoV-2 variants. Given the length of the public health emergency, it is not uncommon for the general public and healthcare professionals to be mentally fatigued [13, 14]. While the level of fear about COVID-19 has generally abated, the risk for SARS-CoV-2 infection and related outcomes remains [15, 16]. One month after ending the global public health emergency, the WHO reported 1.2 million new COVID-19 cases and 7100 deaths between 22 May 2023 and 18 June 2023 [17].
Immunocompromised (IC) populations continue to have a higher risk for severe COVID-19 outcomes, including prolonged hospitalization and death [18,19,20,21,22]. Since vaccination against COVID-19 may be less effective in the IC population, the Centers for Disease Control and Prevention (CDC) currently advises that persons aged ≥ 6 months with moderate to severe immune compromise may receive optional additional boosters in an acknowledgement of their increased risk or need for additional protection [23, 24]. Therefore, while the perception of the waning impact of COVID-19 may have some validity in the general population, IC individuals still face significant risk.
Published data evaluating the economic impact of immunocompromising conditions on the cost of COVID-19 treatment and recovery using real-world evidence is limited and few studies have evaluated the impact of Omicron on immunocompromised populations. A UK-based study conducted in 2022 using electronic health records [25] found that IC populations had higher rates of hospitalizations, ICU admissions, and deaths. Similarly, studies conducted in British Columbia, Canada (January 2022–March 2022) [26], and the Netherlands (December 2021–January 2022) [27] during the early Omicron period also found a disproportionate burden of hospitalization for immunocompromised individuals. The initial results (1 April 2020–31 March 2022) published from this ongoing study demonstrated that IC persons with COVID-19 experienced a high rate of hospitalizations (23.5%) and substantial associated costs (mean $64,029 [2021 USD]) [28]. The current analysis updates the previously published data in several important ways: it provides a cumulative update through the end of 2022 (1 April 2020–31 December 2022); creates a general population cohort for qualitative comparison; and presents hospitalizations, lengths of stay (LOS), and healthcare costs for IC populations and the general population in the year 2022 (i.e., exclusively during the Omicron period) to provide healthcare decision-makers with the most relevant data and observed trends related to COVID-19 and SARS-CoV-2 infection in the IC population.
Methods
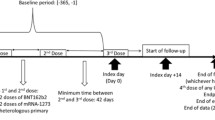
The study design (Fig. 1), study population, methods, and outcomes of this ongoing, retrospective, observational cohort study have been previously published [28]. In brief, this study is being conducted among patients identified in the Healthcare Integrated Research Database (HIRD®), a large database curated by Carelon Research that is built on a broad, clinically rich, and geographically diverse spectrum of longitudinal medical and pharmacy claims from health plan members across the USA. The HIRD contains neighborhood-level characteristics at the census block group level relevant to social determinants of health (SDOH) obtained from the 2019 American Community Survey (ACS) [29]. All data were accessed as a limited data set for which a business associate and data use agreement was in place with the covered entities in compliance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. All protected health data were deidentified and thus exempt from institutional review board review.
Study design. *Use of immunosuppressive medications was assessed starting on 1 October 2019. For all other cohorts, risk was evaluated beginning on 1 April 2018. ‡The index date can occur at any point during the infection period. §Censor date is the earliest health plan disenrollment, death, or the end of the study period. γActive treatment assessment date is the earliest of SARS-CoV-2 infection, member’s health plan disenrollment, death, or end of the study period. Active treatment assessment date can be the same as the censor date (figure shows a case where they differ for illustration purposes)
Cohort Definitions
A diverse group of individuals with both competent and compromised immune systems were included in the overall study population. Patients with ≥ 1 claim for an immunocompromising at-risk condition or procedure or ≥ 2 claims/fills for an immunosuppressive (IS) medication during the study period were included in the appropriate IC cohort(s). The index date for the IC cohorts was defined as the date of diagnosis of an immunocompromising condition or use of an IS treatment in the study period or 1 April 2020 (the first day of the infection period), whichever came last. Patients with ≥ 12 months of continuous pharmacy and medical benefit enrollment prior to the index date were included. The index date for the HIRD cohort was included as the first day after 12 months of continuous enrollment from 1 April 2019 to 31 December 2022. Mutually non-exclusive IC cohorts were actively treated hematologic/solid tumor malignancy (identified as patients with ≥ 1 claim for an antineoplastic medication from 6 months before index date to the date of the COVID-19 diagnosis, member’s health plan disenrollment, death, or end of the study period, whichever came first); hematopoietic stem cell transplant (HSCT)/solid organ transplant (SOT); primary immunodeficiency (PID); chronic kidney disease (CKD) stage 5/dialysis; and IS treatment. A mutually exclusive composite cohort of the overall IC population was also created.
The infection period was defined as 1 April 2020 (the date when the specific ICD-10 diagnosis code for COVID-19 [U07.1] was released in the USA) to 31 December 2022 (updated from 31 March 2022 in the initial analysis). The baseline period was from 1 April 2018 to the index date. The follow-up period was defined as the time from the cohort member’s index date to the date of health plan disenrollment, death, or end of the study period, whichever came first. Different selection criteria for the HIRD and IC cohorts may have resulted in IC individuals included in the HIRD cohort having a different index date and consequently different baseline, and follow-up periods compared to the IC cohort.
Outcomes
Baseline measures included demographic (sex, age on index date, age group, and payor type) and clinical characteristics (Quan-Charlson Comorbidity Index [QCI] score and comorbidities of interest, as well as the categorical number of immunocompromising conditions and IS treatments of interest) [30]. The socioeconomic status (SES) [31] index score is a composite measure based on seven variables (unemployment rate, poverty rate, median household income, median home value, rate of no high school diploma, rate of college degree, and crowding) obtained from ACS 2019 data at the neighborhood level. The SES index score is reported in quartiles, with “4” indicating a patient is in the top 25% of the SES index score (i.e., most advantaged SES group) and “1” indicating a patient is in the bottom 25% of the SES index score (i.e., least advantaged SES group).
For COVID-19 measures, infection was identified on the basis of a positive SARS-CoV-2 polymerase chain reaction (PCR), or antigen test analyzed by accredited laboratories or a medical encounter (inpatient, emergency room [ER], office visit, urgent care, or telehealth visit) for treatment/evaluation and management/consultation with a COVID-19 diagnosis code at any position on the medical claim.
The severity of COVID-19 was classified into the following mutually exclusive categories: severe/critical, moderate, or mild/asymptomatic. Of note, the definitions of severity used in this study are generally aligned with the severity states used in the WHO clinical progression scale [32]. Severe/critical COVID-19 was defined as an inpatient admission for COVID-19 with an intensive care unit (ICU) admission or one of the following: evidence of extracorporeal membrane oxygenation (ECMO), mechanical ventilation use, administration of high-flow oxygen via nasal cannula, or discharge status of expired (scored as 6–9 in the WHO scale, dependent upon the level of support required, or 10 [dead]). Moderate COVID-19 was defined as an inpatient admission for COVID-19 that did not meet any of the criteria for severe/critical COVID-19 (scored as 4–5 in the WHO scale, dependent upon whether low-flow oxygen supplementation was required). Mild COVID-19 was defined as an outpatient claim for COVID-19 (e.g., office, telehealth, urgent care, or stand-alone ER visit) without any inpatient claims (scored as 2–3 in the WHO scale, dependent upon whether assistance was required), while asymptomatic COVID-19 was defined as a positive SARS-CoV-2 lab result with no inpatient or outpatient COVID-19 claims (scored as 1 in the WHO scale). The mild and asymptomatic categories were combined as it was not possible to fully ascertain whether the patient had symptoms but did not seek medical care. COVID-19 vaccination dose was identified using a combination of claims data and immunization information systems reports from the CDC. The presence of at least one COVID-19 vaccine record in 6 months before the infection date was identified irrespective of the type and number of vaccines.
COVID-19 measures included the cumulative incidence and incidence rate (IR) of COVID-19, LOS, and total costs (health plan paid + patient paid) for COVID-19 hospitalization associated with first diagnosis. Relative risk (RR) of hospitalization with first diagnosis of COVID-19 for IC cohorts compared to the HIRD cohort were calculated. All costs were adjusted to 2022 US dollars (USD) according to medical care price index information provided by the US Bureau of Labor Statistics [33].
Statistical Analysis
This study was descriptive, with no statistical comparisons between cohorts. Descriptive statistics were reported as mean and standard deviation (SD) or median and interquartile range (IQR) for continuous data and absolute and relative frequencies for categorical data. The prevalence of immune compromise was calculated as the number of patients with an immunocompromising condition or using an IS treatment in the infection period divided by the total number of patients with pharmacy and medical benefit enrollment in the infection period. The cumulative incidence of COVID-19 was calculated as the number of patients who developed COVID-19 in the infection period divided by the total number of patients within each cohort of interest. Incidence rates of COVID-19, calculated as the number of new cases of COVID-19 during the infection period divided by the total person-years in each cohort during the infection period, were reported with 95% confidence intervals (CIs). Both overall and monthly IRs were calculated. In addition, overall IRs by severity of COVID-19 were assessed. The frequency, LOS, and total all-cause costs of hospitalizations associated with the first COVID-19 diagnosis were stratified by severity and reported for all cohorts. The RR of hospitalization associated with the first diagnosis of COVID-19, calculated as the risk of hospitalization in the IC cohorts divided by the risk of hospitalization in the HIRD cohort, were reported with 95% CIs. Total aggregate costs for COVID-19-related hospitalizations were calculated by multiplying the total number of ICU and non-ICU hospitalized patients by the mean total all-cause costs for ICU and non-ICU hospitalizations, respectively. Sample selection, creation of analytic variables, and analyses were performed using the Instant Health Data (IHD) platform (Panalgo, Boston, MA, USA) and Statistical Analysis System (SAS), version 9.3 (SAS Institute Inc, Cary, NC, USA).
Results
The results obtained from the IC cohorts in the present study, which included an additional 9 months of data when Omicron was the predominant variant, were comparable to those previously published [28]. The disposition of the study population into the HIRD and IC cohorts for the updated study period is displayed in Fig. 2. Within the 25,080,848 individuals in the HIRD (1 April 2018–31 December 2022), the prevalence of immune compromise was 2.9% (N = 736,407), an increase of 0.2% from the initial analysis (2.7%; 1 April 2018–31 March 2022). The prevalence of each immunocompromising condition was similar to the initial analysis, and changes did not exceed ± 0.2% in any IC cohort in the updated analysis (Table 1). The mean number of immunocompromising conditions was 1.2 for the composite IC cohort, compared to 0.1 for the HIRD cohort. The IC cohort with the highest number of immunocompromising conditions was the HSCT/SOT cohort, with an average of 2.4 immunocompromising conditions.
Patient disposition. Updated study period: 1 April 2018 through 31 December 2022. Use of IS treatment was measured between 1 October 2019 and 31 December 2022; the presence of all other immunocompromising conditions was assessed between 1 April 2018 and 31 December 2022. CKD chronic kidney disease, HIRD Healthcare Integrated Research Database, HSCT hematopoietic stem cell transplant, HSTM actively treated hematologic/solid tumor malignancy, IC immunocompromised, IS immunosuppressive, PID primary immunodeficiency, SOT solid organ transplant
Baseline Demographics and Clinical Characteristics
Baseline demographics in the updated study period (Table 2) remained similar to those presented in the initial analysis. The mean age in the IC cohorts ranged from 50.4 years (PID) to 63.2 years (actively treated hematologic/solid tumor malignancy), while the HIRD cohort was younger (mean 37.5 years). Median family income and racial distribution (per SDOH at the Census block level) were fairly consistent across cohorts (including the HIRD). The notable exception was the CKD stage 5/dialysis cohort, which had the lowest family income and highest percentage of black, non-Hispanic, and Hispanic/Latino members across all cohorts. The CKD stage 5/dialysis cohort also had the highest percentage of members with low SES and the lowest rate of members with high SES (Table 2).
Baseline comorbidities in the updated study period (Table 3) were generally similar to the initial analysis. Notable observations of QCI categories show that more than half of the members of three IC cohorts (65.1% of CKD stage 5/dialysis]; 57.3% of actively treated hematologic/solid tumor malignancy; and 54.4% of HSCT/SOT) had 3+ QCI categories, with higher scores associated with increased risk for mortality within a year. In contrast, only 4% of the HIRD cohort had 3+ QCI categories, with a mean QCI score of 0.4. The comorbidity with the highest prevalence across all cohorts was hypertension, ranging from 21.1% (HIRD) to 92.1% (CKD stage 5/dialysis).
Cumulative Incidence of COVID-19
With 9 months of additional data (1 April 2022–31 December 2022), the cumulative incidence of COVID-19 increased between 2.7% and 4.7% across all cohorts (Fig. 2). The cumulative incidence was highest (22.8%) among individuals with CKD stage 5/dialysis and lowest (12.4%) in the HIRD cohort. Those with hematologic/solid tumor malignancies receiving active treatment had a cumulative incidence of 13.7%, while those using IS treatment had a cumulative incidence of 18.3%. Patients with PID and HSCT/SOT had rates of 20.5% and 20.4%, respectively. In the year 2022, the cumulative incidence of COVID-19 in the HIRD cohort was 8.4%, which was lower than the composite IC cohort (13.4%) and all individual IC cohorts (range 10.8–15.9%). A record of a COVID-19 vaccination administration was observed in 10.5–17.8% of patients in the 6 months before the infection date.
Incidence Rate of COVID-19
The IR of COVID-19 in the composite IC cohort increased from 101.3 cases per 1000 patient-years (initial results; 1 April 2020–31 March 2022) to 115.7 cases per 1000 patient-years in the updated infection period (1 April 2020–31 December 2022) (Table 4). In comparison, the overall IR of COVID-19 in the HIRD cohort (unavailable in the initial analysis) was 77.8 cases per 1000 patient-years over the updated infection period. Consistent with the original study, the monthly IR of COVID-19 was highest in the CKD stage 5/dialysis cohort (approximately two to ten times higher than the HIRD cohort) for a majority of the updated study infection period (28 of 33 months; Fig. 3a, b). Most IC cohorts experienced asymptomatic or mild COVID-19 cases two to five times more frequently than moderate to severe cases; the exception was the CKD stage 5/dialysis cohort, in which moderate to severe cases of COVID-19 were more frequent (Table 4). In the individual IC cohorts, IRs of moderate COVID-19 were 4 to 17.7 times higher than the HIRD cohort (11.7–51.3 cases vs 2.9 cases per 1000 patient-years), and IRs of severe COVID-19 were 4 to 35.6 times higher than the HIRD cohort (7.9–67.7 cases vs 1.9 cases per 1000 patient-years).
Healthcare Resource Utilization and Costs Associated with First COVID-19 Hospitalization
Overall, the CKD stage 5/dialysis cohort had the highest percentage of patients with hospitalizations associated with their first COVID-19 diagnosis (59.1%), which was double the next closest IC cohort (HSCT/SOT, 29.0%) and more than ten times higher than the HIRD cohort (4.7%) (Table 5). Compared to the HIRD cohort, RR for hospitalization associated with the first COVID-19 diagnosis ranged from 4.3 (for IS treatment users) to 23.0 (for patients with CKD stage 5/dialysis) in the IC population. Average total all-cause costs (health plan paid + patient paid) associated with the first COVID-19 hospitalization in most IC cohorts were 1.2 to 2.9 times higher than the HIRD cohort ($42,840–101,683 vs $35,649). The exception was the actively treated hematologic/solid tumor malignancy cohort, with a mean of $30,257 for the first COVID-19 hospitalization. Total aggregate costs from April 2020 through December 2022 were approximately $3.8 billion for the HIRD cohort, with approximately $1.2 billion (> 30%) contributed by IC individuals (i.e., the composite IC cohort) who comprise only about 3% of the HIRD population. Furthermore, two-thirds (~ $850 million) of the costs contributed by IC individuals were due to hospitalizations for severe SARS-CoV-2 infections.
Focusing on results for 2022 only (when Omicron was the predominant variant in the USA) shows similar trends to those seen for the updated study infection period (1 April 2020–31 December 2022). While only 3.7% of the HIRD cohort were hospitalized, 46.8% of the CKD stage 5/dialysis cohort, 24.6% of the HSCT/SOT cohort, and 19.5% of the PID cohort were hospitalized, accounting for the highest percentages of hospitalizations associated with first COVID-19 diagnosis (Table 6). Compared to the HIRD cohort, RR for hospitalization associated with the first COVID-19 diagnosis ranged from 5.4 (for IS treatment users) to 21.3 (for patients with CKD stage 5/dialysis). The average LOS for the HIRD and composite IC cohorts were 8.7 days and 11.7 days, respectively, and mean total all-cause costs were $28,848 and $42,719. Total aggregate costs were approximately $1 billion for the HIRD cohort, with the composite IC cohort contributing approximately $310 million (30%). Two-thirds (~ $195 million) of the costs contributed by IC individuals were due to hospitalizations for severe COVID-19 cases.
Discussion
The latest results from the EPOCH-US study provide additional real-world evidence of COVID-19-related outcomes for the IC population and the general (immunocompetent + IC) population, comparable to the commercially insured US population. Immunocompromised patients represented 2.9% of the overall population, consistent with previously published papers [34, 35]. IC cohorts had consistently higher overall and monthly IRs of COVID-19, and severe COVID-19 cases in these patients disproportionately contributed to their higher inpatient utilization and costs.
The cumulative incidence of COVID-19 among the IC cohorts in this study varied, ranging from 13.7% in the actively treated hematologic/solid tumor malignancy cohort to 22.8% in the CKD stage 5/dialysis cohort. The higher cumulative incidence of COVID-19 is likely due to the longer study period (1 April 2020–31 December 2022) and the variability of each variant’s predominance, transmissibility, and virulence over time. Monthly IRs for the HIRD population are lower than those for the IC population during the study period. Differences in the monthly IRs for the HIRD population and IC cohorts are larger between April 2022 and December 2022. Additionally, IRs for moderate and severe COVID-19 were lower in the HIRD cohort than the IC cohorts (~ 11–65 cases more per 1000 patient-years). This trend could be due to the difference in the predominant variants (Omicron and its sub-lineages) coupled with the suboptimal effect of vaccination in IC populations. Even when fully vaccinated, IC individuals may not mount an adequate response to the vaccine. They may have low titers of antibodies with less neutralizing capability that wane more rapidly than in those with intact immune systems [36,37,38,39,40,41]. In contrast to Alpha and Delta variants, Omicron is characterized by significant immune escape and therapeutic monoclonal antibody escape, enabling efficient infection of vaccinated or previously infected individuals [42,43,44,45,46,47]. Available antiviral medications (e.g., nirmatrelvir/ritonavir and molnupiravir) can decrease the risk of progression to severe COVID-19, although each drug comes with related challenges [48, 49]. Omicron and its sub-lineages have been shown to cause reduced COVID-19 severity compared to earlier variants [44, 50]. The updated analysis of this ongoing study indicates that the patient’s immune status likely has some influence on the incidence and severity of COVID-19.
The current analysis focused significantly on hospitalizations, LOS, and costs for the latest full calendar year (2022). As a result of the rapid nature of the evolution of the COVID-19 pandemic, these data are most relevant and informative to healthcare decision-makers within health systems and managed care organizations. In 2022, a larger percentage of patients were admitted to the hospital for their initial COVID-19 diagnosis in the IC cohorts (15.4% for composite IC) compared to the HIRD cohort (3.7%). The hospitalizations linked to first COVID-19 diagnosis in the composite IC cohort had longer LOS (11.7 days vs 8.7 days) and higher average total costs ($42,719 vs $28,848) than those in the HIRD cohort. The one exception we noted was the actively treated hematologic/solid tumor malignancy cohort, with similar LOS (8.7 days) and slightly lower average total costs ($27,349) compared to the HIRD cohort. We feel these results were likely due to the combination of patients with hematologic malignancies and patients with solid tumor malignancies in a single cohort, so we will be evaluating these subgroups separately in a future analysis. Severe cases resulting in hospitalization with ICU admission were the main driver of high mean costs for all groups. This difference is likely due to the increased complexity of the IC population as a result of their underlying health condition(s), which may also complicate their SARS-CoV-2 infection. While mean LOS and costs associated with the first COVID-19 hospitalization decreased in 2022 compared to the entire study infection period, the trends of increased LOS and costs in the IC population, compared to the general population, were maintained. The study found that the CKD stage 5/dialysis group remained a significant outlier within the IC population. Additionally, the incidence rates of infection were highest during Omicron variant prevalence. Consistent with our results, an analysis published in Morbidity and Mortality Weekly Report (MMWR; 11 August 2023) showed that individuals on maintenance dialysis had higher rates of SARS-CoV-2 infection and COVID-19-related death compared to the US population aged > 65 years, particularly during the Omicron variant period [51].
We further explored the disproportionate cost burden of COVID-19 for the year 2022. A small percentage of COVID-19 patients (4.8%) caused a large cost burden (~ 30%) on the healthcare system. Severe cases (39%) accounted for almost two-thirds of the $310 million in hospitalization expenses. Utilizing targeted preventive therapies such as monoclonal antibodies in this small but costly high-risk population has the potential to reduce the clinical burden for these patients, be cost-effective, and improve overall treatment outcomes. A similar trend for significant and disproportionate costs in the IC population for hospitalization and costs for the entire study period was also observed. Although indirect costs were not collected in this study, the impact of missed work, costs to family, long-term COVID-related impact, and delayed or missed non-COVID diagnoses will further add to healthcare utilization and cost burden on the system.
It is essential to place our findings within the context of the study design. The difference in COVID-19 outcomes between the HIRD population and IC populations may also be associated with observed differences in demographic and clinical characteristics. Immunocompromised individuals were older and had more comorbidities than the HIRD population. Experts determined criteria for identifying IC individuals, but there could be others at risk for severe COVID-19 outcomes. More research is needed to determine which subgroups of IC individuals are most vulnerable to COVID-19. Factors such as type of cancer, length of immune compromise, and treatment intensity should be considered to identify those most at risk. This will help target preventive measures that maximize cost-effectiveness and reduce overall healthcare costs. The COVID-19 pandemic placed significant strain on the US healthcare system and caused healthcare worker shortages and resource limitations. A comprehensive understanding of the most vulnerable populations will help alleviate this burden. It is important to prioritize IC individuals, as many in the USA have been vaccinated and/or exposed to SARS-CoV-2 and preventive measures like masks and physical distancing have decreased since the end of the COVID-19 public health emergency in 2023. Despite COVID-19 fatigue, we must recognize and address the ongoing burden experienced by the IC population.
Some limitations of our study are worthy of note. Claims data is mainly collected for payment purposes, not research, so it has limitations when analyzing healthcare outcomes. However, it can still be useful for research purposes. Claims data would not capture COVID-19 testing data that did not initiate a health insurance claim (e.g., federal COVID-19 testing sites, at-home antigen tests) or patients with asymptomatic or mild cases of COVID-19 that did not seek testing or treatment. Additionally, IC individuals may be more likely to be tested for COVID-19 because of the risks associated with their underlying disease or increased interactions within the healthcare system. False positive COVID-19 PCR and antigen tests may have occurred; however, home testing results were not utilized. Claims data are subject to coding errors. To ensure accurate reporting and analysis of COVID-19 cases, the infection period started on 1 April 2020 (coinciding with the release of a specific ICD-10 diagnosis code for COVID-19 [U07.1]). It is possible, however, that a relatively small number of members were infected with SARS-CoV-2 before April 2020. Certain important patient clinical characteristics (e.g., individual-level SDOH, family history, etc.) are not available in claims data and could not be analyzed. The impact of COVID-19 on the severity of underlying conditions cannot be fully understood from claims data alone; however, other researchers have observed the impact of COVID-19 on worsening of underlying neurological conditions [52, 53]. This study did not consider the immunity acquired from a previous infection, which could result in underestimating the occurrence of COVID-19. The use of antiviral medication was subject to evolving regulatory and clinical guidance during the study period. The findings of this study may be generalizable only to patients with health insurance, as SARS-CoV-2 infection rates and healthcare costs in uninsured individuals were not explored. Finally, this analysis only captures data through 31 December 2022 (through the early Omicron [BA.1/BA.2/BA.4/BA.5/BA.2.75/BA.4.6/BA.2.75.2/BQ.1.1] wave) and does not include new variants. Additional data and ongoing analyses will be needed to understand the impact of new variants on the IC population.
Conclusion
According to findings from the EPOCH-US study, individuals with an immunocompromising condition or those receiving IS treatment are at an increased risk of contracting COVID-19 and experiencing severe outcomes, including hospitalization. Despite IC individuals comprising less than 3% of the HIRD population, their hospitalization expenses accounted for approximately 30% of the total costs incurred by individuals hospitalized for their initial COVID-19 diagnosis. Our study indicates that 18.3% of all IC individuals contracted COVID-19, with 15.4% requiring hospitalization. We feel it is important to note the consistent increased risk of severe COVID-19 outcomes seen among the CKD stage 5/dialysis cohort in our study, as they are not traditionally categorized as an IC population. While a trend towards less severe COVID-19 outcomes has been noted in the general population, IC individuals continue to bear a high burden of disease severity and healthcare utilization due to their underlying health condition, comorbidity profile, and potential suboptimal protection from vaccination. Further research is required to identify specific subpopulations at the greatest risk of severe COVID-19 outcomes and to develop targeted interventions to supplement current preventive strategies.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available due to contractual obligations with the data sources.
References
World Health Organization. COVID-19 Weekly Epidemiological Update. Edition 145 published 1 June 2023: World Health Organization; 2023 [updated June 1, 2023]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-june-2023. Accessed 10 Sept 2023.
Centers for Disease Control and Prevention. COVID Data Tracker. Weekly Update for the United States Atlanta, GA: Department of Health and Human Services, CDC; 2023 [updated June 22, 2023]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 30 June 2023.
Centers for Disease Control and Prevention. COVID Data Tracker. Daily Update for the United States Atlanta, GA: U.S. Department of Health & Human Services, CDC; 2023 [updated February 23, 2023]. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 28 Feb 2023.
Walmsley T, Rose A, John R, et al. Macroeconomic consequences of the COVID-19 pandemic. Econ Model. 2023;120:106147.
Hlávka J, Rose A. Covid-19’s total cost to the U.S. economy will reach $14 trillion by end of 2023 [Internet]. (2023). Available from: https://healthpolicy.usc.edu/article/covid-19s-total-cost-to-the-economy-in-uswill-reach-14-trillion-by-end-of-2023-new-research/.
Zabidi NZ, Liew HL, Farouk IA, et al. Evolution of SARS-CoV-2 variants: implications on immune escape, vaccination, therapeutic and diagnostic strategies. Viruses. 2023;15(4):944.
Cao L, Lou J, Chan SY, et al. Rapid evaluation of COVID-19 vaccine effectiveness against symptomatic infection with SARS-CoV-2 variants by analysis of genetic distance. Nat Med. 2022;28(8):1715–22.
Galloway SE, Paul P, MacCannell DR, et al. Emergence of SARS-CoV-2 B.1.1.7 lineage—United States, December 29, 2020-January 12, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(3):95–9.
Duong D. Alpha, Beta, Delta, Gamma: what’s important to know about SARS-CoV-2 variants of concern? Can Med Assoc J. 2021;193(27):E1059.
Ahmad F, Cisewski J, Xu J, Anderson R. Provisional mortality data-United States, 2022. MMWR Morb Mortal Wkly Rep. 2023;72:488–92.
Centers for Disease Control and Prevention. End of the Federal COVID-19 Public Health Emergency(PHE) Declaration Atlanta, GA: Centers for Disease Control and Prevention; 2023 [updated May 5, 2023]. https://www.cdc.gov/coronavirus/2019-ncov/your-health/end-of-phe.html. Accessed 10 Sept 2023.
World Health Organization. Coronavirus Disease (COVID-19) Pandemic: Overview: World Health Organization; 2023 [updated June 21, 2023]. https://www.who.int/europe/emergencies/situations/covid-19. Accessed 10 Sept 2023.
Coaston J. The new phase of the pandemic is Covid exhaustion: we’re over covid. Are we able to move on from it for good? New York Times. 2022 March 9, 2022.
Wadman M. When is a pandemic “over”? Science. 2022;375(6585):1077–8.
Cleveland Clinic. No, the COVID-19 pandemic isn’t over: ending the U.S. public health emergency delcaration doesn’t mean COVID-19 is gone. Cleveland, OH: Cleveland Clinic; 2023. https://health.clevelandclinic.org/is-the-pandemic-over/. Accessed 10 Sept 2023.
Rathke B, Yu H, Huang H. What remains now that the fear has passed? Developmental trajectory analysis of COVID-19 pandemic for co-occurrences of Twitter, Google Trends, and public health data. Disaster Med Public Health Prep. 2023;17:e471.
World Health Organization. COVID-19 Weekly Epidemiological Update. Edition 148 published 22 June 2023: World Health Organization; 2023 [updated June 22, 2023]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-june-2023. Accessed 11 Sept 2023.
Abbasi J. Researchers tie severe immunosuppression to chronic COVID-19 and virus variants. JAMA. 2021;325(20):2033–5.
Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137–45.
Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms. Am J Emerg Med. 2021;45:378–84.
SeyedAlinaghi S, Abbasian L, Solduzian M, et al. Predictors of the prolonged recovery period in COVID-19 patients: a cross-sectional study. Eur J Med Res. 2021;26(1):41.
Velayos FS, Dusendang JR, Schmittdiel JA. Prior immunosuppressive therapy and severe illness among patients diagnosed with SARS-CoV-2: a community-based study. J Gen Intern Med. 2021;36(12):3794–801.
Hofsink Q, Haggenburg S, Lissenberg-Witte BI, et al. Fourth mRNA COVID-19 vaccination in immunocompromised patients with haematological malignancies (COBRA KAI): a cohort study. EClinicalMedicine. 2023;61:102040.
Moulia DL, Wallace M, Roper LE, et al. Interim recommendations for use of bivalent mRNA COVID-19 vaccines for persons aged ≥ 6 months—United States, April 2023. MMWR Morb Mortal Wkly Rep. 2023;72(24):657–62.
Evans RA, Dube S, Lu Y, et al. Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study. Lancet Reg Health Eur. 2023;35:100747.
Bahremand T, Yao JA, Mill C, Piszczek J, Grant JM, Smolina K. COVID-19 hospitalisations in immunocompromised individuals in the Omicron era: a population-based observational study using surveillance data in British Columbia, Canada. Lancet Reg Health Am. 2023;20:100461.
Malahe SRK, Hoek RAS, Dalm VASH, et al. Clinical characteristics and outcomes of immunocompromised patients with coronavirus disease 2019 caused by the omicron variant: a prospective observational study. Clin Infect Dis. 2022;76(3):e172–8.
Ketkar A, Willey V, Pollack M, et al. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: the EPOCH-US Study. Curr Med Res Opin. 2023;39(8):1103–18.
U.S. Census Bureau. Community Survey 5-Year Data Profile (2015–2019) Washington, DC: U.S. Census Bureau; 2019. https://www.census.gov/programs-surveys/acs/data.html. Accessed 11 Sept 2023.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82.
Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev. 2008;29(3):27–42.
WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e7.
U.S. Bureau of Labor Statistics. CPI Inflation Calculator Washington, DC: U.S. Bureau of Labor Statistics; 2023. https://www.bls.gov/data/inflation_calculator.htm. Accessed 11 Sept 2023.
Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316(23):2547–8.
Wallace BI, Kenney B, Malani PN, Clauw DJ, Nallamothu BK, Waljee AK. Prevalence of immunosuppressive drug use among commercially insured US adults, 2018–2019. JAMA Netw Open. 2021;4(5):e214920-e.
Agha ME, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect Dis. 2021;8(7):ofab353.
Cheung MW, Dayam RM, Shapiro JR, et al. Third and fourth vaccine doses broaden and prolong immunity to SARS-CoV-2 in adult patients with immune-mediated inflammatory diseases. J Immunol. 2023;211(3):351–64.
Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–2.
Simon B, Rubey H, Treipl A, et al. Haemodialysis patients show a highly diminished antibody response after COVID-19 mRNA vaccination compared with healthy controls. Nephrol Dial Transplant. 2021;36(9):1709–16.
Tartof SY, Slezak JM, Puzniak L, et al. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg Health Am. 2022;9:100198.
Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccines for preventing coronavirus disease 2019 hospitalizations in the United States. Clin Infect Dis. 2022;74(9):1515–24.
Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–63.
Khan K, Karim F, Ganga Y, et al. Omicron BA.4/BA.5 escape neutralizing immunity elicited by BA.1 infection. Nat Commun. 2022;13(1):4686.
Peacock TP, Brown JC, Zhou J, et al. The altered entry pathway and antigenic distance of the SARS-CoV-2 Omicron variant map to separate domains of spike protein. Preprint. BioRxiv. 2022:2021.12.31.474653.
Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185(14):2422–3313.
Willett BJ, Grove J, MacLean OA, et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat Microbiol. 2022;7(8):1161–79.
Willett BJ, Kurshan A, Thakur N, et al. Distinct antigenic properties of the SARS-CoV-2 Omicron lineages BA.4 and BA.5. Preprint. BioRxiv. 2022:2022.05.25.493397.
De Vito A, Colpani A, Saderi L, et al. Impact of early SARS-CoV-2 antiviral therapy on disease progression. Viruses. 2022;15(1):71.
Rahmah L, Abarikwu SO, Arero AG, et al. Oral antiviral treatments for COVID-19: opportunities and challenges. Pharmacol Rep. 2022;74(6):1255–78.
Suzuki R, Yamasoba D, Kimura I, et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature. 2022;603(7902):700–5.
Jose Navarrete GB. SARS-CoV-2 infection and death rates among maintenance dialysis patients during delta and early omicron waves—United States, June 30, 2021–September 27, 2022. MMWR Morb Mortal Wkly Rep. 2023;2023(72):871–6.
Amruta N, Chastain WH, Paz M, et al. SARS-CoV-2 mediated neuroinflammation and the impact of COVID-19 in neurological disorders. Cytokine Growth Factor Rev. 2021;58:1–15.
Sakibuzzaman M, Hassan A, Hayee S, et al. Exacerbation of pre-existing neurological symptoms with COVID-19 in patients with chronic neurological diseases: an updated systematic review. Cureus. 2022;14(9):e29297.
Medical Writing, Editorial, and Other Assistance
Writing and editorial assistance in the preparation of this article was provided by Mrs. Elizabeth Marks, BS, of Carelon Research. Operational support for the preparation of this article was provided by Ms. Claire Bocage, MPH, of Carelon Research. Both were funded by AstraZeneca.
Funding
Sponsorship for this study, the journal Rapid Service Fee, and the Open Access Fee were funded by AstraZeneca.
Author information
Authors and Affiliations
Contributions
Conceptualization/design of the study: Amita Ketkar, Vincent Willey, Lisa Glasser, Monica Verduzco-Gutierrez, Dennis Cunningham; Material preparation, data acquisition, and analysis of the data: Amita Ketkar, Vincent Willey, Cachet Wenziger, Chia-Chen Teng. All authors contributed to the interpretation of the data, drafting of the manuscript or revising it critically for intellectual content, and approved the final version of the manuscript to be published. All authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of Interest
Christine Dube, Lisa Glasser, and Sunny Hirpara are employees and shareholders of AstraZeneca. Monica Verduzco-Gutierrez has received honoraria and travel to give lectures related to long COVID-19; unrelated to this work, she has been a consultant with AbbVie (consultant/advisor, speakers bureau), Merz (consultant/advisor, speakers bureau), Ipsen (grant/research, consultant/advisor, speakers bureau), Medtronic (consultant/advisor), and Piramal (speakers bureau). Dennis Cunningham has been a consultant/advisor for AstraZeneca. Dennis Cunnigham had an affiliation with Henry Ford Health, Detroit, MI, USA at the time of study which has changed since to Michigan State University, MI, USA. Carelon Research received funding from AstraZeneca to conduct this study. Amita Ketkar, Vincent Willey, Cachet Wenziger, and Chia-Chen Teng are employees of Carelon Research (formerly known as HealthCore). Amita Ketkar and Vincent Willey are shareholders of Elevance Health (formerly Anthem), which is the parent company of Carelon Research (formerly HealthCore). Casey Dobie is an employee of Xcenda, a Cencora company, and a consultant for AstraZeneca.
Ethical Approval
Ethics committee approval was not required for this study. All data were accessed as a limited data set for which a business associate and data use agreement was in place with the covered entities in compliance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. All protected health data were deidentified and thus exempt from institutional review board review.
Additional information
Prior Publication: This manuscript is an update of the prior study by Ketkar A, Willey V, Pollack M, et al. (Curr Med Res Opin 39:1103–18, 2023).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ketkar, A., Willey, V., Glasser, L. et al. Assessing the Burden and Cost of COVID-19 Across Variants in Commercially Insured Immunocompromised Populations in the United States: Updated Results and Trends from the Ongoing EPOCH-US Study. Adv Ther 41, 1075–1102 (2024). https://doi.org/10.1007/s12325-023-02754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02754-0