Abstract
Purpose
Many believe that blood pressure management during cardiac surgery is associated with postoperative outcomes. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to determine the impact of high compared with low intraoperative blood pressure targets on postoperative morbidity and mortality in adults undergoing cardiac surgery on cardiopulmonary bypass (CPB). Our primary objective was to inform the design of a future large RCT.
Source
We searched MEDLINE, EMBASE, Web of Science, CINAHL, and CENTRAL for RCTs comparing high with low intraoperative blood pressure targets in adult patients undergoing any cardiac surgical procedure on CPB. We screened reference lists, grey literature, and conference proceedings.
Principal findings
We included eight RCTs (N =1,116 participants); all examined the effect of blood pressure management only during the CPB. Trial definitions of high compared with low blood pressure varied and, in some, there was a discrepancy between the target and achieved mean arterial pressure. We observed no difference in delirium, cognitive decline, stroke, acute kidney injury, or mortality between high and low blood pressure targets (very-low to low quality evidence). Higher blood pressure targets may have increased the risk of requiring a blood transfusion (three trials; n = 456 participants; relative risk, 1.4; 95% confidence interval, 1.1 to 1.9; P = 0.01; moderate quality evidence) but this finding was based on a small number of trials.
Conclusion
Individual trial definitions of high and low blood pressure targets varied, limiting inferences. The effect of high (compared with low) blood pressure targets on other morbidity and mortality after cardiac surgery remains unclear because of limitations with the body of existing evidence. Research to determine the optimal management of blood pressure during cardiac surgery is required.
Study registration
PROSPERO (CRD42020177376); registered: 5 July 2020.
Résumé
Objectif
Pour beaucoup, la prise en charge de la pression artérielle pendant la chirurgie cardiaque serait associée aux issues postopératoires. Nous avons réalisé une revue systématique et une méta-analyse d’études randomisées contrôlées (ERC) afin de déterminer l’impact de cibles peropératoires de pression artérielle élevées par rapport à des cibles basses sur la morbidité et la mortalité postopératoires d’adultes bénéficiant d’une chirurgie cardiaque sous circulation extracorporelle (CEC). Notre objectif principal était d’orienter la conception d’une future ERC d’envergure.
Sources
Nous avons analysé les bases de données MEDLINE, EMBASE, Web of Science, CINAHL et CENTRAL afin d’en tirer les ERC comparant des cibles de pression artérielle peropératoire élevées à des cibles basses chez des patients adultes bénéficiant d’une intervention chirurgicale cardiaque sous CEC. Nous avons passé au crible les listes de références, la littérature grise et les travaux de congrès.
Constatations principales
Nous avons inclus huit ERC (N = 1116 participants); toutes les études ont examiné l’effet de la prise en charge de la pression artérielle uniquement pendant la CEC. Les définitions d’une pression artérielle élevée ou basse variaient d’une étude à l’autre et, dans certains cas, un écart a été noté entre la pression artérielle cible et la pression artérielle moyenne atteinte. Nous n’avons observé aucune différence dans les taux de delirium, de déclin cognitif, d’accident vasculaire cérébral, d’insuffisance rénale aiguë ou de mortalité entre les cibles de pression artérielle élevée et basse (données probantes de qualité très faible à faible). Des cibles de pression artérielle plus élevées pourraient avoir augmenté le risque de transfusion sanguine (trois études; n = 456 participants; risque relatif, 1,4; intervalle de confiance à 95 %, 1,1 à 1,9; P = 0,01; données probantes de qualité modérée), mais ce résultat se fondait sur un petit nombre d’études.
Conclusion
Les définitions individuelles des cibles d’hypertension et d’hypotension artérielle variaient, ce qui a limité les inférences. L’effet de cibles de pression artérielle élevée (par rapport à une pression artérielle basse) sur d’autres mesures de la morbidité et de la mortalité après une chirurgie cardiaque demeure incertain en raison des limites de l’ensemble des données probantes existantes. Des recherches visant à déterminer la prise en charge optimale de la pression artérielle pendant la chirurgie cardiaque sont nécessaires.
Enregistrement de l’étude
PROSPERO (CRD42020177376); enregistrée le 5 juillet 2020.
Similar content being viewed by others
Blood pressure during cardiac surgery is a key parameter that is controlled by anesthesiologists before and after cardiopulmonary bypass (CPB) and facilitated by perfusionists during CPB. Clinicians manage intraoperative blood pressure using vasopressors, inotropes, vasodilators, anesthetic agents, pump flow changes during CPB, and intravascular volume administration. Generally, clinicians believe that optimal management of blood pressure during cardiac surgery is important because of an assumed relationship with postoperative morbidity and mortality. Nevertheless, available evidence is limited, so what constitutes “optimal” blood pressure management during cardiac surgery remains poorly defined. As a result, the blood pressure maintained during cardiac surgery is largely based on institutional preference,1 often with higher (or lower) targets chosen for patients with certain comorbidities. The use of low, high, and personalized blood pressure targets has been described.
Many clinicians believe that a lower mean arterial pressure (MAP) in the range of 40–60 mmHg is associated with end-organ hypoperfusion and resultant ischemia; therefore, higher targets (≥ 70 mmHg) are preferable. This belief has been inconsistently supported by observational studies and several small randomized controlled trials (RCTs) in cardiac surgery that have suggested a relationship between low MAP and an increased risk of adverse outcomes.2,3,4,5 In contrast, other clinicians target this lower blood pressure range (e.g., MAP 40–60 mmHg) because they believe that higher targets may be associated with their own harms, which is supported by limited observational and RCT data.6,7 A third group support a personalized approach to blood pressure management based on the concept of autoregulation (principally cerebral), where constant blood flow to an organ is maintained across a range of perfusion pressures. Because the blood pressure range within which end-organ blood flow is kept constant may be influenced by individuals’ baseline blood pressure, pre-existing hypertension, and the presence of obstructive vascular disease, this approach establishes intraoperative blood pressure targets based on the preoperative assessment of baseline blood pressure8 or cerebral autoregulation thresholds.9
Considering the uncertainty as to the optimal approach, we conducted a systematic review and meta-analysis of RCTs to assess the impact of high compared with low blood pressure targets during cardiac surgery on CPB on postoperative morbidity and mortality. Our objective was to inform the design of future RCTs evaluating the use of intraoperative blood pressure management as an intervention.
Methods
We registered the protocol for this systematic review with PROSPERO International Prospective Register of Systematic Reviews (CRD42020177376). Our review was conducted according to the guidelines recommended by the Cochrane Handbook10 and is reported according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines (see Electronic Supplementary Material [ESM], eAppendix 1: PRISMA checklist).11
Trial eligibility criteria
We included RCTs comparing high with low intraoperative blood pressure targets in adult patients undergoing cardiac surgery on CPB; within-patient crossover and quasi-randomized studies were excluded. We examined the postoperative outcomes of delirium, cognitive decline, stroke, myocardial infarction (MI), acute kidney injury, blood transfusion, intensive care unit (ICU) length of stay (LOS), hospital LOS, all-cause mortality, and quality of life. We accepted outcomes as defined by the trial.
Identification of trials
Our search strategy was designed and conducted with the support of a health sciences librarian (J.Y.). We searched MEDLINE, EMBASE, Web of Science, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and the Cochrane Register of Controlled Trials (CENTRAL) from inception to 7 May 2021, to identify potentially relevant publications (see ESM eAppendix 2 for detailed search terms). We reviewed reference lists of included trials, abstracts from major conferences from the preceding five years (American Society of Anesthesiologists, Society of Cardiovascular Anesthesiologists, European Association of Cardiothoracic Anesthesiologists, and European Society of Cardiology), and searched clinical trials registries (ClinicalTrials.gov, International Standard Randomised Controlled Trial Number Register, Australian New Zealand Clinical Trials Registry, and World Health Organization International Clinical Trials Registry Platform) to identify relevant publications. When potentially relevant clinical trials registry entries without associated publications were identified, we contacted investigators to determine the status of the trial and whether preliminary data were available.
Selection of trials
Five reviewers (C.M., Y.Q., T.A., E.B.C., J.S.) screened titles and abstracts for eligibility using Covidence, an online platform for systematic review management (https://www.covidence.org; Covidence, Melbourne, VIC, Australia).12 We retrieved full texts of included trials and reviewed each full text to determine whether it met inclusion criteria. When relevant, we identified the primary reason for exclusion. We performed all steps independently and in duplicate; disagreements between reviewers were resolved by discussion. If the discussion failed to resolve disagreement, a third reviewer was consulted to make a final decision.
Data extraction
We developed and piloted a standardized data extraction form. Four reviewers (C.M., Y.Q., T.A., H.K.) independently extracted data about the trial intervention and outcomes; a third reviewer (M.K., E.B.C., or J.S.) checked for incongruency and resolved conflicts. Along with general trial information, we extracted trial characteristics (e.g., trial design, data collection process, follow-up period), participant and intervention characteristics (e.g., age and sex distribution of participant groups, procedure types, mean duration of CPB), and outcomes reported, including how the outcome was defined. We contacted authors to obtain relevant unreported data. We attempted contact twice, after which we considered the data missing.
Assessment of risk of bias
Six reviewers (C.M., Y.Q., T.A., H.K., M.K., J.S.) independently and in duplicate assessed the risk of bias of included trials using the Cochrane risk of bias tool.13 We assessed the following domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. We considered trials to have a “high risk of bias” if they had a high risk of bias in a single domain or unclear risk of bias in two or more domains. Any incongruencies in risk of bias assessment were resolved by discussion.
Data analysis
We conducted analyses using Review Manager 5.3 software (Cochrane Collaboration, London, UK).14 We used a random effects model to pool results and summarize evidence. When trials reported outcomes at multiple timepoints, we meta-analyzed the outcome assessment reported closest to hospital discharge only unless otherwise specified. We present point estimates as relative risks (RRs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes, with 95% confidence intervals (CI) and corresponding P values. When continuous outcomes were reported as median and interquartile range [IQR], we used validated methods to convert to mean and standard deviation (SD) for the purposes of calculating a pooled effect estimate.15 We constructed forest plots to describe available evidence according to the method described by DerSimonian and Laird.16 For the outcome of blood transfusion incidence, we made a post hoc adjustment using the Hartung–Knapp method, a correction that can be applied to random effects meta-analysis to minimize type I error when the number of included studies is small.17 We assessed the heterogeneity of data for each outcome using I2 statistics with the thresholds recommended by Cochrane for interpretation.18
Subgroup analyses
We a priori planned to undertake subgroup analyses to explain statistical heterogeneity and to assess subgroup effects. Our planned subgroup analyses included the following: high risk of bias compared with low risk of bias trials, older (≥ 75 yr) compared with younger patients, patients with vascular risk factors compared with those without, type of surgery, and timing of follow-up. If sufficient trials were identified, we planned to compare trials where MAP targets were applied during the entire intraoperative period with studies only targeting MAP during CPB.
Publication bias
We planned to use a funnel plot or the Egger test to assess for publication bias if more than ten trials were identified.
Assessment of quality of evidence
We assessed the quality of the evidence for each outcome using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach,19 which considers within-trial risk of bias, consistency (or heterogeneity) of the data, directness of the evidence, precision of effect estimates, and the presence of possible reporting bias.
Results
Literature search
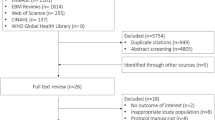
We identified 6,370 records and, after removal of duplicates, screened the titles and abstracts of 4,467 studies. Following screening of titles and abstracts, 59 studies were included for full text review. Of these, we excluded 51 studies—19 were duplicates (conference abstracts or substudies of already included trials); four were clinical trial entries (contact with investigators confirmed that two studies were incomplete, one had been stopped before data had been collected, and one had generated a publication that was included); three were the wrong population (one animal study and two studies in nonsurgical heart failure patients);6,20,21 13 were not RCTs; and 12 used the wrong intervention. Three of the 12 RCTs using the “wrong intervention” reported the effects of an intraoperative blood pressure management strategy on patient outcomes but were excluded because the intervention arms could not be categorized as “high” compared with “low” (see ESM eAppendix 3: Blood pressure RCTs using a different intervention strategy). We included eight RCTs (N = 1,116 participants);4,5,22,23,24 two of these RCTs reported outcomes in additional substudy publications (see ESM eAppendix 4: List of sub-study publications).6,25 The study flow diagram is presented in Fig. 1.
Characteristics of included trials
Table 1 summarizes the characteristics of included trials. Most patients underwent elective coronary artery bypass grafting (CABG); some trials included urgent and emergent cases as well as more complex cardiac procedures. The MAP targets used across trials are shown in Fig. 2. Individual trial definitions of high and low targets were 40–70 mmHg in low arms and 70–100 mmHg in high arms; the pooled mean (SD) high and low achieved MAPs were 70 (9) and 54 (9) respectively, not including one trial that did not report the achieved blood pressure.23 Two trials compared a high MAP target to a “usual care” arm without a formally stated target.21,24 Trial definitions of high compared with low blood pressure overlapped significantly and, in some trials, there was a discrepancy between the target MAP and the actual MAP achieved. Sirvinskas et al.20 divided patients into three blood pressure ranges (low, middle, and high pressure); for the purposes of meta-analysis, we included outcomes reported for the high and low target arms and excluded the “middle” group.
Target and achieved mean arterial pressure (MAP) by intervention arm within each trial. Point with solid vertical line represents achieved mean and standard deviation blood pressure. Hatched vertical line represents target blood pressure range. High, low, and middle range MAP targets are represented in red, blue, and green respectively. *Trials with no specified low blood pressure range.
Data collection
The data extracted for each trial, as well as details regarding how it was handled, are described in ESM eAppendix 5. The corresponding author for one trial provided unpublished mean with SD values for ICU and hospital LOS, which had originally been reported as median with IQR.6
Risk of bias
The risk of bias of each included trial is illustrated in Fig. 3, and detailed assessment rationale are available in ESM eAppendix 6. Two trials had a low risk of bias5,22 and six had a high risk of bias.4,6,20,21,23,24 Four high risk of bias trials were at high risk of reporting or attrition bias.6,2021,23 Two high risk of bias trials were at high risk of selection bias.21,24 Three high risk of bias trials had high risk of performance and detection bias.4,6,23
Assessment of quality of evidence
The GRADE assessment is presented in ESM eAppendix 7; the overall quality of evidence for each outcome is specified below. In general, most outcomes were rated down because of the high risk of bias of included trials, high statistical heterogeneity, and a confidence interval that included both significant benefit and significant harm.
Outcomes
Table 2 presents the pooled effect estimate for each outcome; associated forest plots can be found in ESM eAppendix 8. Due to the small number of trials and limited data reported for each outcome, we were unable to conduct subgroup analyses or assess for publication bias.
Neurocognitive outcomes
We found no difference in the incidence of delirium (two trials; n = 289 participants; RR, 0.5; 95% CI, 0.03 to 8.6; P = 0.61; very low quality evidence),4,6 cognitive decline (two trials; n = 394 participants; RR, 1.2; 95% CI, 0.7 to 2.1; P = 0.46; very low quality evidence),5,6 or stroke (three trials; n = 527 participants; RR, 1.0; 95% CI, 0.2 to 4.9; P = 0.95; very low quality evidence)5,6,24 when high compared with low intraoperative blood pressure targets were used. Four trials could not be meta-analyzed because of the way in which outcomes were reported. Azau et al. reported no difference between low and high blood pressure target arms in the incidence of “neurologic complications of surgery,” which was a composite that included stroke, seizure, and transient mental confusion or agitation.22 Sirvinskas et al. reported no difference between low, middle, and high blood pressure arms in the incidence of postoperative “neurologic disorders,” an outcome that was not defined.20 Bagheri et al. reported no difference between low and high target groups in mean postoperative cognitive test scores.23 Siepe et al. reported a greater decline in postoperative Mini Mental Status Exam in the low compared with the high blood pressure target arm (mean [SD] 3.9 [6.1] vs 1.1 [1.9]; P = 0.012).4
Myocardial infarction
Myocardial infarction was reported in three trials (n = 734 participants), with an overall event rate of 2.0% (15/734).5,6,22 There was no difference in MI (RR, 0.9; 95% CI, 0.3 to 3.2; P = 0.89; low quality evidence) when high compared with low intraoperative blood pressure targets were used.
Acute kidney injury
The number of patients that required postoperative hemodialysis was reported by five trials (n = 740 participants), with no significant difference between high and low MAP target (RR, 1.0; 95% CI, 0.5 to 2.3; P = 0.98; low quality evidence).4,6,20,22,24 The number of patients meeting RIFLE “risk” criteria was reported by four trials (n = 600 participants); there was no significant difference when high compared with low intraoperative blood pressure targets were used (RR, 1.3; 95% CI, 0.9 to 1.8; P = 0.25; very-low quality evidence).6,21,22,24
Bleeding/transfusion
Three trials reported the incidence of transfusion (n = 456 participants);4,20,22 one reported this as the number of patients who received transfusion of red blood cells20 and two reported the number of patients who received blood products.4,22 Using random effects meta-analysis, patients managed using a high target blood pressure were more likely to be transfused than those in low blood pressure arms (RR, 1.4; 95% CI, 1.1 to 1.9; P < 0.01; moderate quality evidence). We were surprised at this finding and, post hoc, applied a Hartung–Knapp adjustment, a correction that can be applied to random effects meta-analysis to minimize type I error when the number of included studies is small.17 Doing so resulted in a wider CI that no longer achieved statistical significance (RR, 1.4; 95% CI, 0.8 to 2.5; P = 0.10). There was no difference in the mean number of units transfused when high compared with low intraoperative blood pressure targets were used (three trials; n = 632 participants; MD, 0.1 units; 95% CI, -0.1 to 0.3; P = 0.35; high quality evidence).4,5,22
Length of stay
Intensive care unit LOS was reported by four trials (n = 609 participants).4,5,6,20 While most trials separated ICU LOS from all other levels of care, Siepe et al. reported time spent in ICU and “intermediate care” as a single measure. When meta-analyzed, there was no difference in ICU LOS between high and low blood pressure groups (MD, 0.2 days; 95% CI, -0.4 to 0.8; P = 0.55; very-low quality evidence). Hospital LOS was 1.1 days longer when high compared with low intraoperative blood pressure targets were used (five trials; n = 901 participants; MD, 1.1 days; 95% CI, 0.3 to 1.8; P < 0.01; low quality evidence).4,5,6,20,22 Nevertheless, the quality of evidence for this outcome was rated down for indirectness, as the results of one trial22 had to be converted from median [IQR] to mean (SD), which likely resulted in a falsely narrow confidence interval for the results of both that individual trial and the point estimate for the pooled effect.
Mortality
Mortality was reported by four trials (n = 671 participants) with a pooled event rate of 5.1% at six months (34/671).4,5,6,24 The use of high compared with low intraoperative blood pressure targets did not result in a difference in mortality, whether in hospital (RR, 1.1; 95% CI, 0.4 to 3.3; P = 0.84; very low quality evidence), at 30 days after surgery (RR, 1.6; 95% CI, 0.6 to 4.4; P = 0.33; low quality evidence) or six months after surgery (RR, 0.8; 95% CI, 0.4 to 1.6; P = 0.55; moderate quality evidence).
Quality of life
Quality of life was reported by one trial (n = 248 participants). Gold et al. used the SF-36 to assess quality of life at baseline and six months after surgery. They reported that most patients improved in all seven domains with no difference between high and low blood pressure arms in quality of life or decline in physical function at six months after surgery as measured by the SF-36.5
Discussion
Eight RCTs enrolled 1,116 adult patients undergoing cardiac surgery on CPB and assessed the effect of high compared with low intraoperative blood pressure targets on postoperative morbidity and mortality. Based on low–moderate quality evidence, high compared with low intraoperative blood pressure targets did not result in a demonstrable difference in delirium, cognitive decline, stroke, MI, acute kidney injury, ICU LOS, hospital LOS, or all-cause mortality, despite the positive results in some of the individual trials included in this meta-analysis. Higher blood pressure targets were associated with a 40% increase in the risk of transfusion (moderate quality evidence) using random effects meta-analysis. Nevertheless, when we undertook a post hoc analysis using a more conservative statistical method, this finding was no longer statistically significant. Hospital LOS was 1.1 days longer when high compared with low intraoperative blood pressure targets were used. Nevertheless, because the results of one trial22 had to be converted from median [IQR] to mean (SD), we had limited confidence in this finding.
Several observational studies have examined the impact of intraoperative blood pressure throughout the entire surgical period on postoperative outcomes of cardiac surgery. Reich et al. conducted a retrospective study of 2,149 patients undergoing CABG and identified a 30% increase in the odds of in-hospital mortality in patients with increased time during CPB with a MAP below 50 mmHg.26 Aronson et al. conducted a retrospective study of 7,808 patients undergoing cardiac surgery, and found that every minute during surgery above or below a systolic blood pressure of 105–130 mmHg was associated with a 3% increase in the odds of 30-day mortality (odds ratio [OR], 1.03 per minute; 95% CI, 1.02 to 1.39; P < 0.0001). Sun et al. found that a sustained MAP of less than 64 mmHg during CPB was associated with postoperative stroke. Every ten minutes that MAP was between 55 and 64 mmHg was associated with a 13% increase in the odds of stroke (adjusted OR [aOR], 1.13; 95% CI, 1.05 to 1.21) and every ten minutes that MAP was less than 55 mmHg was associated with a 16% increase in the odds of stroke (aOR, 1.16; 95% CI, 1.08 to 1.23). While these studies had relatively large sample sizes, all were derived from single centres, which limits the generalizability of their findings. Furthermore, no RCTs have gone on to evaluate whether implementing strategies to avoid the extremes of blood pressure associated with risk in these observational studies decreases postoperative morbidity and mortality.
Other work examining the relationship between intraoperative MAP and postoperative outcomes has examined the effect of individualized blood pressure targets on postoperative morbidity and mortality. In a single-centre RCT of 412 patients undergoing CABG, Charlson et al. compared the use of a “high” MAP (80 mmHg) target with the use of a custom MAP based on patients’ baseline blood pressure and found no difference in mortality, deterioration in quality of life, and major neurologic, cardiac, and cognitive complications. Using target based proprietary hardware and software that calculated the individualized lower limit of cerebral autoregulation,9 Brown et al. conducted a nested substudy27 of a larger trial of individualized blood pressure management,28 and found that the incidence of postoperative delirium was 37.9% when an individualized blood pressure target was used, compared with an incidence of 52.7% among patients who received usual care (OR, 0.55; 95% CI, 0.31 to 0.97; P = 0.04).27 Nevertheless, there was no difference between these two groups in the primary composite neurologic outcome of clinical stroke, new ischemic lesions on postoperative diffusion-weighted magnetic resonance imaging, or cognitive decline from baseline to four to six weeks after surgery in either the Brown substudy27 or its parent trial conducted by Hogue et al.28 Furthermore, the technique used to determine the lower limit of cerebral autoregulation is specialized and requires access to technology that is not widely available, which limits its applicability in routine clinical practice.
The effect of blood pressure management on morbidity and mortality has been studied in settings other than cardiac surgery, with varying results. A recent systematic review of observational studies including 130,862 patients undergoing noncardiac surgery examined the relationship between intraoperative hypotension (using variable definitions of what constituted hypotension) and postoperative morbidity and mortality.29 The authors found that intraoperative hypotension was associated with an increased risk of morbidity (OR, 2.1; 95% CI, 1.6 to 2.8) and mortality (OR, 1.9; 95% CI, 1.3 to 2.8).29 In contrast, in the ICU setting, an individual patient data meta-analysis of 894 patients with septic shock found no difference in the odds of 28-day mortality when higher compared with lower MAP targets were used (OR, 1.2; 95% CI, 0.9 to 1.5).30 Nevertheless, when patients in the higher MAP groups had been on vasopressors for more than six hours prior to randomization, they had a higher risk of death (OR, 3.0; 95% CI,1.3 to 6.7).30 Based on this finding, the authors hypothesized that the administration of elevated doses of vasopressors to maintain blood pressure at higher target levels may be harmful when used for prolonged periods.30
With considerable uncertainty as to what constitutes the optimal blood pressure during cardiac surgery, professional bodies have issued conflicting recommendations for blood pressure management. In their consensus statement concerning the management of intraoperative blood pressure, the Perioperative Quality Initiative—an international organization aiming to improve patient care by developing consensus statements related to perioperative medicine—recommended that intraoperative systolic blood pressure be maintained below 140 mmHg and stated that injury was a function of arterial pressure severity and duration.31 No recommendation was made with respect to a lower limit of blood pressure. In contrast, guidelines from the European Association of Cardiothoracic Anesthesiologists/European Association of Cardiothoracic Surgeons on the management of CPB recommend that MAP during CPB be maintained between the broad range of 50–80 mmHg; no recommendation was made for blood pressure management before and after CPB.32
Our systematic review is limited by the available evidence. Our search strategy and inclusion criteria identified eight small, single-centre RCTs that were eligible for inclusion. Across RCTs, the definition of what constitutes a “high” compared with a “low” target varied, such that “low” and “high” targets overlapped across trials (Fig. 2). In addition, there was often a discrepancy between the target and achieved blood pressure. For example, the “low” blood pressure range of 60–70 mmHg targeted by Siepe et al.4 overlapped with the “high” value of > 60 mmHg targeted by Kandler et al.,24 as well as the achieved “high” blood pressure mean (SD) of 66.8 (4.9) mmHg observed by Vedel et al. (despite a “high” blood pressure target range of 70–80 mmHg).6 This limits our ability to make inferences about the relative effects of these two approaches. Ideally, rather than dichotomizing blood pressure targets as “high” and “low,” we would undertake an individual patient meta-analysis, which would allow us to characterize the relationship between blood pressure (characterized continuously) and postoperative morbidity and mortality. This would prevent the loss of power that occurs because of dichotomization. Unfortunately, even given access to patient-level data for each included trial, the small number of trials and patients would be unlikely to provide adequate power to do so. Furthermore, because included RCTs only considered blood pressure during the CPB period, blood pressure management before or after CPB may have diminished the observed impact of “high” compared with “low” blood pressure targets on postoperative morbidity and mortality. Finally, in conducting our meta-analysis, we did not adjust for multiple comparisons. Nevertheless, only one of our results was statistically significant using a threshold P = 0.05; we have limited confidence in this result (for hospital LOS) because it involved the conversion of median [IQR] to mean (SD). Thus, adjusting for multiple comparisons would not have changed our interpretation of our findings. The results of our systematic review highlight the gaps in the existing literature and provide the basis for future work examining the relationship between intraoperative blood pressure and major morbidity and mortality after cardiac surgery.
Conclusions
The effect of high compared with low blood pressure targets on morbidity and mortality after cardiac surgery remains unclear because of limitations in existing evidence. Research to determine the optimal management of blood pressure during cardiac surgery is required.
Change history
02 February 2022
This article was updated to correct Hessam Kashani’s name.
14 February 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12630-022-02205-4
References
Murphy GS, Hessel EA 2nd, Groom RC. Optimal perfusion during cardiopulmonary bypass: an evidence-based approach. Anesth Analg 2009; 108: 1394-417.
Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014; 147: 483-9.
Rudiger A, Begdeda H, Babic D, et al. Intra-operative events during cardiac surgery are risk factors for the development of delirium in the ICU. Crit Care 2016; DOI: https://doi.org/10.1186/s13054-016-1445-8.
Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg 2011; 40: 200-7.
Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg 1995; 110: 1302-11.
Vedel AG, Holmgaard F, Rasmussen LS, et al. High-target versus low-target blood pressure management during cardiopulmonary bypass to prevent cerebral injury in cardiac surgery patients: a randomized controlled trial. Circulation 2018; 137: 1770-80.
Aronson S, Stafford-Smith M, Phillips-Bute B, et al. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology 2010; 113: 305-12.
Charlson ME, Peterson JC, Krieger KH, et al. Improvement of outcomes after coronary artery bypass II: a randomized trial comparing intraoperative high versus customized mean arterial pressure. J Card Surg 2007; 22: 465-72.
Liu X, Akiyoshi K, Nakano M, et al. Determining thresholds for three indices of autoregulation to identify the lower limit of autoregulation during cardiac surgery. Crit Care Med 2021; 49: 650-60.
Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from URL: www.training.cochrane.org/handbook (accessed November 2021).
Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; DOI: https://doi.org/10.1186/2046-4053-4-1.
Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia. Available from ULR: www.covidence.org (accessed November 2021).
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; DOI: https://doi.org/10.1136/bmj.d5928.
The Cochrane Collaboration. Review Manager (RevMan). Computer Program, Version 5.4. 2020.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; DOI: https://doi.org/10.1186/1471-2288-14-135.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177-88.
IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol 2014; DOI: https://doi.org/10.1186/1471-2288-14-25.
Deeks JJ, Higgins JP, Altman DG; Cochrane Statistical Methods Group. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, et al. (Eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from URL: training.cochrane.org/handbook (accessed November 2021).
Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924-6.
Sirvinskas E, Benetis R, Raliene L, Andrejaitiene J. The influence of mean arterial blood pressure during cardiopulmonary bypass on postoperative renal dysfunction in elderly patients. Perfusion 2012; 27: 193-8.
Urzua J, Troncoso S, Bugedo G, et al. Renal function and cardiopulmonary bypass: effect of perfusion pressure. J Cardiothorac Vasc Anesth 1992; 6: 299-303.
Azau A, Markowicz P, Corbeau JJ, et al. Increasing mean arterial pressure during cardiac surgery does not reduce the rate of postoperative acute kidney injury. Perfusion 2014; 29: 496-504.
Bagheri K, Motamedi O, Aghavoudi O, Akbari M. The effects of mean arterial pressure during cardiopulmonary bypass on clinical and paraclinical parameters during and after coronary artery bypass graft surgery. J Isfahan Med Sch 2012; 29: 1-10.
Kandler K, Nilsson JC, Oturai P, et al. Higher arterial pressure during cardiopulmonary bypass may not reduce the risk of acute kidney injury. J Cardiothorac Surg 2019; DOI: https://doi.org/10.1186/s13019-019-0929-4.
Larsen MH, Draegert C, Vedel AG, et al. Long-term survival and cognitive function according to blood pressure management during cardiac surgery. A follow-up. Acta Anaesthesiol Scand 2020; 64: 936-44.
Reich DL, Bodian CA, Krol M, Kuroda M, Osinski T, Thys DM. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesth Analg 1999; 89: 814-22.
Brown CH 4th, Neufeld KJ, Tian J, et al. Effect of targeting mean arterial pressure during cardiopulmonary bypass by monitoring cerebral autoregulation on postsurgical delirium among older patients: a nested randomized clinical trial. JAMA Surg 2019; 154: 819-26.
Hogue CW, Brown CH 4th, Hori D, et al. Personalized blood pressure management during cardiac surgery with cerebral autoregulation monitoring: a randomized trial. Semin Thorac Cardiovasc Surg 2021; 33: 429-38.
Wijnberge M, Schenk J, Bulle E, et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open 2021; DOI: https://doi.org/10.1093/bjsopen/zraa018.
Lamontagne F, Day AG, Meade MO, et al. Pooled analysis of higher versus lower blood pressure targets for vasopressor therapy septic and vasodilatory shock. Intensive Care Med 2018; 44: 12-21.
Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth 2019; 122: 563-74.
Authors/Task Force Members; Kunst G, Milojevic M, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Br J Anaesth 2019; 123: 713-57.
Author information
Authors and Affiliations
Contributions
Charlotte C. McEwen, Emilie P. Belley-Coté, and Jessica Spence contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Hilary Grocott, David Mazer, Scott Brudney, Eric Jacobsohn, Richard P. Whitlock contributed to study conception and design. Takhliq Amir, Yuan Qiu, Jack Young, Hessam Kashani, Morvarid Kavosh, Anne Vedel, and Eugene Wang contributed to the acquisition of data. Takhliq Amir, Yuan Qiu, Kevin Kennedy, Hessam Kashani, and Morvarid Kavosh contributed to the analysis of data. Kevin Kennedy contributed to the interpretation of data.
Corresponding author
Ethics declarations
Disclosures
None.
Funding statement
Dr. Spence is supported by a Clinician Scientist Award from the Canadian Anesthesia Research Foundation. Dr. Belley-Côté is supported by a National New Investigator Award from the Heart and Stroke Foundation.
Editorial responsibility
This submission was handled by Dr. Stephan K.W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was updated to correct Hessam Kashani’s name.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
McEwen, C.C., Amir, T., Qiu, Y. et al. Morbidity and mortality in patients managed with high compared with low blood pressure targets during on-pump cardiac surgery: a systematic review and meta-analysis of randomized controlled trials. Can J Anesth/J Can Anesth 69, 374–386 (2022). https://doi.org/10.1007/s12630-021-02171-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-02171-3