Abstract
The acceptability and feasibility of large-scale testing with lateral flow tests (LFTs) for clinical and public health purposes has been demonstrated during the COVID-19 pandemic. LFTs can detect analytes in a variety of samples, providing a rapid read-out, which allows self-testing and decentralized diagnosis. In this Review, we examine the changing LFT landscape with a focus on lessons learned from COVID-19. We discuss the implications of LFTs for decentralized testing of infectious diseases, including diseases of epidemic potential, the ‘silent pandemic’ of antimicrobial resistance, and other acute and chronic infections. Bioengineering approaches will play a key part in increasing the sensitivity and specificity of LFTs, improving sample preparation, incorporating nucleic acid amplification and detection, and enabling multiplexing, digital connection and green manufacturing, with the aim of creating the next generation of high-accuracy, easy-to-use, affordable and digitally connected LFTs. We conclude with recommendations, including the building of a global network of LFT research and development hubs to facilitate and strengthen future diagnostic resilience.
Key points
-
Lateral flow tests (LFTs) were adopted at an unprecedented scale during the COVID-19 pandemic, enabling access to testing beyond healthcare settings.
-
Only 0.4% of the 3 billion COVID-19 tests performed through to mid-2022 were conducted in low-income regions, raising ethical concerns and constraining our collective ability to respond to a pandemic.
-
Key barriers to COVID-19 LFT development and adoption include lack of access to well characterized samples, limited accuracy, lack of connectivity, lack of evidence of cost-effectiveness, regulatory delays and centralized manufacturing capabilities.
-
LFTs could also play an important part in the detection of other diseases of epidemic potential and antimicrobial resistance.
-
Bioengineering approaches, such as the use of nano- and quantum materials, nucleic-acid-based LFTs, CRISPR and machine learning, will improve the sensitivity, specificity, multiplexing and connectivity features of LFTs.
-
We recommend investing in an international LFT research and development hub network to spearhead the development of a pipeline of innovative bioengineering approaches to design next-generation LFTs.
Similar content being viewed by others
Introduction
Diagnostics have emerged as a crucial countermeasure to the spread of COVID-19, and by late 2022, more than 3 billion tests for SARS-CoV-2 had been conducted worldwide1. Reverse transcription-polymerase chain reaction (RT-PCR) remains the gold standard for diagnosing COVID-19, and genomic sequencing has become vital for tracking variants. However, lateral flow tests (LFTs), albeit less sensitive than PCR, have enabled an unprecedented scale of global testing in clinical and public health, owing to their simplicity, low cost, accessibility, rapid results and ability to detect infectiousness2 (Fig. 1a).
a, Characteristics of pre-COVID-19 lateral flow tests (LFTs), LFTs deployed in the COVID-19 pandemic, and next-generation LFTs. b, Timeline of key advances in lateral flow testing. WHO, World Health Organization; HIV, human immunodeficiency virus; RDT, rapid diagnostic test; AMR, antimicrobial resistance.
The bioengineering underpinnings of LFTs (also known as rapid diagnostic tests (RDTs), lateral flow assays, lateral flow immunoassays or immunochromatographic tests) date back decades. The first latex agglutination and immunoassays in the 1950s3 and subsequent refinement of the solid-phase lateral flow assay in the 1980s4,5 led to the first LFT pregnancy tests, which were revolutionary in empowering women to manage their own health (Fig. 1b). By the 1990s, the first malaria LFTs were being used by trained healthcare providers, although it took two decades before the pre-qualification requirements of the World Health Organization (WHO) were settled. LFTs have since been developed to diagnose infectious diseases in primary healthcare settings worldwide, including for malaria, human immunodeficiency virus (HIV), Strep A (group A Streptococcus) and influenza A/B, and selected LFTs are now available for self-testing at clinics and pharmacies worldwide. In 2016, the WHO recommended HIV self-testing with LFTs, based on their effectiveness to reach key populations and increase case detection; nonetheless, adoption remains limited6. Compared to other infectious diseases, for which LFT development can take years, SARS-CoV-2 antigen LFTs were developed and deployed within months (Fig. 1b). In 2022, the WHO ‘strongly endorsed’ COVID-19 self-testing with antigen LFTs7, putting the public at the heart of the public health response.
The simplicity of LFTs comes with technical limitations and usage trade-offs. Notably, they are less sensitive than PCR and rely on visual readout. LFTs also lack digital connectivity for data collection and linkage to care. However, innovations in ultra-sensitive nanomaterials, clustered regularly interspaced short palindromic repeats (CRISPR)-based detection, mobile app connectivity and deep learning have greatly improved LFT technology, albeit often at an early stage of technological readiness, reflecting a disconnect between bioengineering research priorities and practical use cases.
In this Review, we discuss the design principle of LFTs, and highlight key lessons learned from their use in the COVID-19 pandemic, including access, accuracy, affordability, manufacturing, regulation and funding8. We examine the implications of decentralized LFT testing for pandemics, endemic infections and antimicrobial resistance, and discuss bioengineering approaches aimed at meeting the REASSURED criteria (that is, having real-time connectivity, ease of sample preparation, being affordable, sensitive, specific, user-friendly, robust and reliable, equipment-free or environmentally friendly, and deliverable to end-users)9. Finally, we summarize research and development (R&D) priorities for researchers, industry, funders and policymakers.
Lateral flow tests
Target analytes and samples
LFTs can be designed to target different analytes, such as antigens (for example, SARS-CoV-2 nucleoproteins) and antibodies (IgG or IgM) (Fig. 2a). LFTs can also detect nucleic acids, although such tests are not commercially available, except in China and from a single US company10. LFTs can detect analytes in blood, urine, saliva or vaginal swabs, with sampling protocols (sample collection, buffers, incubation time) varying by disease, sample matrix and analyte11.
a, A typical lateral flow test (LFT) is composed of a cellulose sample pad, which absorbs the sample, a glass fibre conjugate pad, which stores dried nanoparticle–receptor conjugates, a nitrocellulose membrane with a test line of immobilized capture receptors, and an absorbent pad to wick the sample. The sample is dropped onto the sample pad, and flows down the strip by capillary action into the conjugate pad, where it resuspends the nanoparticle–antibody complex, which binds to the target analyte. These complexes flow into the nitrocellulose and continue to the test line, which is printed with antibodies that bind to a different paratope of the nucleoprotein. The control line is functionalized with antibodies that bind to the antibodies on the nanoparticles, or an alternative species. b, Assay designs for different analyte types. c, The different interactions between the analyte, the detection receptors on the nanoparticle and the capture receptors on the membrane are illustrated (not to scale). Association (ka) and dissociation (kd) rates are enumerated for different affinity binding reactions in LFTs. d, The test is housed in a plastic cassette with a well for sample addition (S), internal contact points to guide flow, and a readout window with test (T) and control (C) line markings. Some tests have a QR code and an identification (ID) number (COVID-19 only). e, COVID-19 LFT kits typically contain a nitrocellulose test membrane strip with dried nanoparticles bearing detector receptors (typically antibodies) on a glass-fibre conjugate pad, housed in a plastic cassette with a QR code and an ID number (note that most pre-COVID-19 LFTs lack these); a nasal swab (for anterior nares or mid turbinate), typically a flocked, rayon or Dacron swab with a polypropylene shaft; an extraction tube containing a solution to extract viral antigens; a plastic waste bag; and written guidance for use, including links to further information or instruction videos. Ag, antigen. Part a adapted with permission from ref. 184, Royal Society of Chemistry, and adapted from ref. 96, Springer Nature Limited, and adapted from ref. 15, Annual Reviews. Part c adapted from ref. 15, Annual Reviews.
Flow
In LFT-based diagnostics, the sample is first placed onto a cellulose sample pad, and then travels by capillary force to the conjugate pad, where previously dried nanoparticle–receptor complexes are resuspended in the sample buffer (Fig. 2a). Here, gold or latex nanoparticles are most frequently used owing to their ease of manufacture, low cost, wide availability, stability, ease of functionalization with proteins, and in the case of gold, strong plasmonic absorption12. In addition, magnetic beads, nanodiamonds13, quantum dots and other particles have been explored14. Mass transport is governed by flow, diffusion and dispersion owing to membrane porosity, but is typically flow-dominated. Flow in LFTs can be described by four flow regimes15; alternatively, Washburn and Darcy equations16 can be applied to model the flow.
Detection
As the sample flows, the target analyte forms complexes with ‘detector’ receptors on the nanoparticles. Once the complexes reach the test line, which is typically printed with a second ‘capture’ receptor that is electrostatically bound to the membrane17, the analyte is bound in a ‘sandwich’ (Fig. 2b). The accumulation of nanoparticles at the test line generates the signal. Here, binding is limited by target–receptor reaction kinetics rather than by mass transport (Fig. 2c). A control line binds the nanoparticles with or without analyte complexation, verifying that the sample has flowed appropriately and that detection complex molecules are functional.
Results
Most LFTs are read qualitatively by visual inspection after 5–30 minutes (Fig. 2d). Alternatively, fluorescent nanoparticles can be used for detection, which may require readers, adding cost, but standardizing results and reducing error owing to subjective interpretation. In addition, quantitative readout data can be captured18,19.
Commercial kit components and users
A typical LFT kit contains a nitrocellulose membrane strip with dried nanoparticles bearing detector receptors on a glass-fibre conjugate pad, housed in a plastic cassette with a QR code and an identification (ID) number (Fig. 2d). In addition, LFT kits designed for nasopharyngeal samples contain a collection swab, typically a flocked, rayon or Dacron tip on a polypropylene shaft, an extraction tube containing a buffer to extract the target antigens, a plastic waste bag, and written guidance for use, including links to further information or videos. LFTs can be administered by trained health professionals (‘professional use’ tests), or self-administered (‘self-tests’).
Lessons learned from COVID-19
Large-scale testing
LFTs were adopted on an unprecedented scale during the COVID-19 pandemic, demonstrating their feasibility and acceptability on a global basis. LFTs have had multiple clinical and public health use cases20,21, such as testing to confirm diagnosis in symptomatic individuals, testing to screen asymptomatic individuals with known exposures or in high-risk groups, such as healthcare workers, care home (elder home) workers, or first responders, screening of asymptomatic individuals at schools, workplaces or mass gatherings, air, land or sea border testing to slow the introduction of new variants, testing to determine the effectiveness of anti-viral treatment, testing for surveillance, and infection-control-based testing in healthcare facilities to facilitate flow of patients22,23.
Professional use and self-tests have enabled LFT-based testing to be expanded beyond healthcare facilities and into community settings and homes (Fig. 3a). COVID-19 testing programmes have been implemented on a city scale (for example, the United Kingdom Liverpool Community testing pilot)24, and on a national scale (for example, nationwide testing in Slovakia)25. In England, 20 million tests were used in less than 12 months, outpacing RT-PCR testing26 (Fig. 3b).
a, The map shows the global distribution of regions in which a COVID-19 self-testing policy was in place, was being considered or was being piloted as of March 2022, adapted from the World Health Organization (WHO)7. b, Rapid adoption of lateral flow tests (LFTs) in England, following their introduction in 2021, surpassing polymerase chain reaction (PCR) use. Data from the UK Coronavirus Dashboard26. c, LFT sensitivity in comparison to PCR. LFT sensitivity aligns with the infectious period of COVID-19 and can detect COVID-19 one to two days after PCR can. The low-cost, portable and rapid format of LFTs allows more frequent testing.
In many high-income regions, COVID-19 self-tests have been widely available since 2021, often subsidized or free to the public through pharmacies or online ordering. A 2022 WHO survey found that COVID-19 self-testing policies have been in place or under consideration in 101 countries7 (Fig. 3a). Self-tests have been used in population surveillance studies, such as the Real-time Assessment of Community Transmission (REACT)-2 study in the UK27, and have been widely accepted and preferred for self-testing in Europe and the USA28,29,30, demonstrating safe and error-free use, as well as correct interpretation of results31,32,33,34. In low- and middle-income regions, COVID-19 self-tests have also shown high acceptability35,36 and close agreement between results from professional use and self-testing (in Malawi and Zimbabwe)7, mirroring earlier findings by the Self-Testing Africa (STAR) initiative on HIV self-testing37. The WHO survey found that regions implementing COVID-19 LFT self-testing perceived many benefits, including more timely diagnosis and self-isolation, increased access to testing and uptake in the population, increased testing frequency, increased adherence to public health and social distancing measures, decreased transmission, and earlier return to pre-COVID-19 activities7, as compared to regions that had not implemented LFT self-testing. However, access to self-tests remains inequitable, with substantially lower adoption in low- and middle-income regions (Fig. 3a and Box 1).
Despite wide use and acceptability, COVID-19 LFTs and the care pathways in which they are used have limitations, particularly in terms of false positives and false negatives (Supplementary Table 1). Concerns (in particular, in low- and middle-income regions) include limited educational interventions, inadequate service delivery models for vulnerable populations, inequities in access, unclear regulations alongside inadequate WHO Emergency Use Listing38, low-quality tests, variability between tests39 and sampling sites40, data loss for public health surveillance, coercive testing, contrived results, unclear guidance for managing positive results, and lack of confirmatory testing. Therefore, more rigorous implementation research is needed, including trials evaluating the clinical effectiveness and cost effectiveness of different LFT-based testing strategies and algorithms.
Accuracy
The accuracy and, in particular, the sensitivity of LFTs is lower than that of reference RT-PCR methods, ranging between 34.1% and 88.1% for SARS-CoV-2 antigen LFTs, with an overall specificity of 99.6% (here, data from instructions-for-use-compliant evaluations in symptomatic participants were used), with sensitivity varying between brands41. Analytically, rapid antigen tests can detect virus at levels equivalent to approximately 100,000 to 1,000,000 SARS-CoV-2 viral genome copies per millilitre39, whereas molecular methods, such as RT-PCR, can detect 1–100 copies per millilitre, and thus, the presence of SARS-CoV-2 at 24–48 hours before LFTs turn positive. Such a trade-off between sensitivity and simplicity has long limited the use of LFTs for certain pathogens. The success of COVID-19 antigen LFTs can be in part attributed to the pathophysiology of SARS-CoV-2 (Fig.3c), that is, its short incubation period and high transmission rates, which are well suited to rapid, frequent testing2,42. In addition, pre-symptomatic and asymptomatic people generally shed sufficiently high antigen loads from nasal and throat samples for timely LFT detection. Moreover, the high antigen load of infectious individuals (established by viral culture) correlates well with COVID-19 LFT analytical sensitivity and specificity2,43. Owing to the long tail of SARS-CoV-2 infection, viral RNA can remain detectable long after live SARS-CoV-2 can no longer be cultured from patient samples, that is, during the non-infectious recovery phase. In addition to being ‘overly sensitive’ in establishing infectiousness, molecular test methods are problematic for large-scale, high-frequency testing programmes, given the need to send samples to centralized laboratories, challenges in scaling-up laboratory capacity, and subsequent delays in receiving test results (which can take days).
Thus, LFTs benefit COVID-19 testing in identifying infectiousness or risk of transmission. LFT testing has enabled healthcare workers to return to work, schools and workplaces to reopen, and economic recovery, including mass gatherings, border testing and travel testing. The viral load threshold for transmission has been proposed to be about 1,000,000 copies44, and therefore, rapid antigen tests are considered to be a good public health tool with which to identify infectious people and those at risk of transmitting SARS-CoV-2 to others, thus reducing community transmission45, with the advantages of ease of use, lower cost, rapid turnaround, and the ability to enable serial daily or weekly testing, which is not currently feasible using RT-PCR testing2. In a pandemic, rapid diagnosis of disease can offset loss of sensitivity, allowing the implementation of public health measures, such as self-isolation and contact tracing, without delay in interrupting the chain of transmission.
The WHO has established a target product profile46 for COVID-19 antigen LFTs for use in suspected COVID-19 cases and close contacts, highlighting the application of LFTs in areas where reference molecular testing is unavailable, or where molecular turnaround times obviate their utility. Specifically, the WHO recommends more than 80% sensitivity (the probability of a positive test, conditioned on truly being positive) and more than 97% specificity (the probability of a negative test, conditioned on truly being negative) for LFTs, using an authorized molecular test (that is, authorized for emergency use by the WHO or the US Food and Drug Administration (FDA)) as reference. Independent evaluations of hundreds of commercial LFTs have been conducted, many supported by the Foundation for Innovative New Diagnostics (FIND)47,48,49, the Paul Ehrlich Institute50, and other public health authorities43.
Even though LFT sensitivity correlates well with infectiousness, false-negative COVID-19 LFT test results remain an issue, particularly early in an infection, when false-negative tests can lead to inadvertent high-risk contacts and ongoing transmission. Therefore, the timing and frequency of LFT testing are important in early symptomatic infection and in screening individuals (before travel or mass gatherings), because infectious individuals may have tested negative by LFT in the prior 24 hours.
Antigen LFT performance and utility also vary with prevalence45,51, requiring careful policies and different testing strategies in different epidemiologic settings; for example, self-isolation and repeated testing in high-prevalence, high-vulnerability settings may be warranted for symptomatic individuals even with a negative COVID-19 LFT result, whereas confirmatory RT-PCR testing may be warranted in low-prevalence settings. LFT accuracy also varies slightly for different COVID-19 variants, because of mutations and pathophysiology changes52. Despite slight differences in sensitivity, most LFTs remain effective in detecting the major variants of concern, including the Delta53 and Omicron54 variants, which contain most mutations in the genes encoding the spike (S) protein, whereas most antigen tests use the nucleocapsid (NP) protein as target.
Fortuitously, COVID-19 antigen LFTs have sufficient accuracy for effective large-scale testing of SARS-CoV-2. However, LFT platforms developed for COVID-19 cannot automatically be transferred to other diseases of epidemic potential, many of which will be more difficult to detect by LFT.
Development and scale-up
Diagnostics have long been underfunded and underused in global health. From the start of the COVID-19 pandemic, a huge amount of funding was directed to SARS-CoV-2 test development and uptake. The US Rapid Acceleration of Diagnostics (RADx) programme invested more than US$1.5 billion (2020) in diagnostics, including for the development of new diagnostics to boost existing laboratory capacity55. The UK government spent an estimated £13.9 billion to make testing freely available between Q2 2020 and Q2 2021 (ref. 56). Member states requested that the WHO prepare a strategy to support access to diagnostics57, testing and vaccines in low- and middle-income regions, which led to the establishment of the access to COVID-19 tools accelerator (ACT-A)1. FIND and the Global Fund, alongside the WHO, co-convened the ‘ACT-A Diagnostics Pillar’, which supported independent evaluation of SARS-CoV-2 antigen LFTs performance, emergency authorization and multiple programmes to increase access to COVID-19 testing in the Global South, including negotiated ceiling prices for SARS-CoV-2 LFTs and RT-PCR kits (Box 1).
RT-PCR tests can be rapidly developed for a new pathogen based on shared sequence data. By contrast, antigen LFT development requires weeks to months in the best of circumstances, including the design of capture receptors (typically antibodies) against target analytes. In addition, companies typically develop their own proprietary reagents, often based on recombinant antigens. Unsurprisingly, international standardization of diagnostic reagents has been problematic during the pandemic. Reference measurement frameworks and standard development help to ensure accurate diagnostics and their availability during an outbreak of a new pathogen. Moreover, established standards can fast-track our understanding of disease pathogenesis by providing comparability of test results.
Millions of LFTs can be produced per month to meet global demand at affordable prices; however, such scale-up requires investment in manufacturing infrastructure and time. Lack of LFT manufacturing capacity was a major COVID-19 response bottleneck until the end of 2020. Coupled with the higher costs of molecular assays and the required instruments and infrastructure, many places lacked sufficient testing capacity in the pandemic’s initial months57. That said, a major pandemic achievement was the timeline to develop, scale and deploy new LFTs for a previously unknown virus, which was ultimately compressed from several years to months (Fig. 1b). The first commercial antigen LFT received emergency use authorization in May 2020 (ref. 58), five months after the first COVID-19 case was reported. Many LFT manufacturers claim that the development timeline could have been even shorter, noting that SARS-CoV-2 is relatively straightforward for antigen detection. The main bottlenecks were access to samples for test optimization and validation, and slow regulatory processes59 (Box 2). In a G7 report tasking policy makers to enable LFT readiness in 100 days60 for the next outbreak, diagnostics manufacturing capacity and regulation were identified as key areas for improvement.
Differing resources, national regulatory requirements, purchase mechanisms, logistics and policy approaches led some regions to adopt LFTs at large scale sooner than others—especially high-income regions. LFT costs and uptake have varied by country during the pandemic, from free tests through government subsidies to end-user prices as high as US$20 per test7. Some low- and middle-income regions experienced difficulties in accessing tests once high-income regions had bought up supply (a problem also seen for COVID-19 vaccines), despite the importance of LFTs in settings with limited molecular testing capacity and rural populations. Regional manufacturing and logistical capabilities for LFT supply became a global concern, given minimal test manufacturing capacity in Africa and elsewhere61. Importantly, funding made available for LFT development and manufacturing during the COVID-19 pandemic could be lost62, but will be required if the world aims to meet the challenge of having LFTs ready in 100 days for the next pandemic, and to address underlying supply chain issues affecting diagnostic access globally63,64.
Digital data capture
COVID-19 LFT results from self-testing, positive or negative, are often not reported65, leaving test use data and true case counts unknown, thereby complicating surveillance; for example, only 14% of LFT results up until the end of May 2021 were reported to UK Test and Trace65. Digital technologies have been deployed throughout the pandemic response66, but opportunities for digital LFT data capture, quality assurance, linkage to care, and resource planning were largely missed (Fig. 4). Public health agencies have been slow to adopt digital innovations, with the first WHO guidelines on digital health interventions for health system strengthening published in 2019 (ref. 67).
a, A generalized schema of clinical user journeys, following a lateral flow test (LFT) in a variety of settings. Not all steps will be required for all conditions (such as COVID-19, malaria and human immunodeficiency virus (HIV)). Steps that are specific to certain types of infection and not relevant to all are shown in red. For example, contact tracing and quarantine are required for COVID-19, but not for malaria. Post-exposure prophylaxis is important in some diseases for high-risk groups (for example, post-exposure prophylaxis for HIV, and oseltamivir for influenza). There is good evidence that LFT user pathways effectively link patients to care, particularly following a positive test result for conditions such as malaria and HIV185. b, The concept of a future m-Health system including an automated LFT classifier and data capture and transmission to a secure m-Health database. Beyond LFT data capture, care and surveillance systems (for example, District Health Information System 2 (DHIS2)) data could be linked to laboratory information systems, stock supply management, staff training and LFT quality control. RDT, rapid diagnostic test. Part b reprinted from ref. 19, Springer Nature Limited.
In the USA, several FDA-authorized LFTs have a companion app, through which the user manually enters test results. LFTs can also contain an integrated reader to detect fluorescent signals and digitize results. In pilot programmes, digital LFTs were provided for travellers entering the USA at certain airports, with voluntary, app-enabled reporting to the US Centers for Disease Control and Prevention68. However, most commercial LFTs provide only a qualitative visual output. Test line intensity depends on multiple factors; in particular, low SARS-CoV-2 antigen concentrations can cause faint test lines, which may be wrongly interpreted as a negative result, risking transmission and a loss of public trust.
Digital approaches to interpreting LFT results have been rare69,70, and have not yet been widely operationalized. A UK research team at i-sense, in partnership with the Africa Health Research Institute, developed an image library of 11,000 field-acquired HIV LFT photographs and deep learning models to classify results for quality assurance. This approach reduces the number of false positives and negatives, compared to visual audit by nurses and community health workers19. The same models were applied to COVID-19 LFTs in partnership with the UK REACT study, and a workflow was developed to analyse more than 500,000 COVID-19 antibody LFT self-tests71. Alternatively, machine learning has been applied to analyse LFTs for UK National Health Service (NHS) staff on a smaller dataset72. These image datasets are taken in real-world conditions and contain weak positives and invalid tests on a variety of devices, enabling more robust classification.
Re-imagining lateral flow tests
LFTs may make a difference in detecting a range of other infections73, particularly the WHO’s list of priority diseases of epidemic potential, antimicrobial resistance and other acute and chronic infections.
WHO priority diseases of epidemic potential
The development and evaluation of diagnostics for diseases of epidemic potential are often only funded during outbreaks, and are sometimes abandoned once the outbreak abates, leaving regions ill-prepared for the next pandemic74. In 2015, in response to the Ebola outbreak in West Africa, the WHO convened experts to develop an R&D blueprint for action to prevent epidemics75, focusing on emerging diseases with the potential to generate a public health emergency, and for which no or insufficient tools existed, aiming at reducing the time between identification of a nascent outbreak and approval of countermeasures (Supplementary Table 2). Commercial LFTs are currently not available for four of the eight known priority diseases of epidemic potential: Crimean Congo haemorrhagic fever, Middle East respiratory syndrome coronavirus (MERS-CoV), Nipah and other henipaviruses, and Rift Valley fever. For the remaining four, bioengineering challenges remain to be addressed. Low sensitivity limits the use of filovirus LFTs (for example, Ebola); thermal stability is needed for Lassa fever LFTs; and Zika LFTs may need to be multiplexed to detect both antigen and IgM to improve specificity. Moreover, ‘disease X’, referring to a serious global epidemic caused by an unknown pathogen, will necessitate an even more agile approach to LFT development and preparedness76.
Industry has historically been reluctant to invest in the development and commercialization of LFTs for pathogens of pandemic potential, owing to an uncertain market size (even during outbreaks), and inconsistent or zero demand in the case of no outbreaks. In addition, well characterized specimens, essential for test development, are often difficult to access. Moreover, performance studies required for regulatory approval are costly. Prior to SARS-CoV-2, progress had been made to mitigate these challenges, for example, in the EU-funded ZikaPlan. Moreover, biobank networks have been set up by the Africa Centres for Disease Control and Prevention77 and FIND. Importantly, diagnostic standards78 need to be established to save product development time, and public health needs must be addressed by research76, including the design of multiplex tests to diagnose undifferentiated fevers at the primary care level, tests co-created with end-users, usable and effective self-tests, and data capture systems for result reporting.
Antimicrobial resistance
The ‘silent pandemic’ of antimicrobial resistance continues to be a substantial global burden, further exacerbated by the COVID-19 pandemic, because screening and surveillance capacity for resistant bacteria gave way to COVID-19 services. Globally, an estimated 4.95 million (3.62–6.57 million) deaths were associated with bacterial antimicrobial resistance in 2019 (ref. 79), and the highest death rates attributable to resistance were in Western sub-Saharan Africa, with 27.3 deaths per 100,000 (20.9–35.3; ref. 79), disproportionately affecting those unable to access expensive second-line antimicrobials80. The Review on Antimicrobial Resistance (commonly known as the O’Neill report) highlights that by 2050, ten million lives a year and a cumulative US$100 trillion of economic output are at risk owing to the rise of drug-resistant infections in the absence of action to reduce antimicrobial resistance81.
The COVID-19 pandemic has reduced public access to care, and antimicrobial prescribing and childhood immunizations have decreased82,83. The number of people treated for drug-resistant tuberculosis declined by 15% in the pandemic’s first year, and global spending on tuberculosis testing, treatment and prevention services dropped by US$500 million (ref. 84). The pandemic’s true impact on global antimicrobial resistance is yet to be confirmed, and new surveillance data must be gathered to update national strategies.
Current methods of determining antimicrobial resistance and susceptibility often rely on bacterial culture, with phenotypic susceptibility testing requiring 36- to 72-hour turnaround times after sample collection, which is too slow for effective antibiotic stewardship in emergency settings or short clinic visits85. The time-to-result can be reduced by rapid, low-cost, point-of-need diagnostics, including by multiplex LFTs with data capture. Priority antimicrobial resistance use cases for LFTs include tests to differentiate bacterial and viral infections, and tests to diagnose sexually transmitted infections (for example, Neisseria gonorrhoeae and Chlamydia trachomatis). However, the optimal biomarker panels for these diagnostics are often not known; genotypic markers do not always reflect phenotypic behaviour, and the most relevant resistance mutations can change over time and by geography85, presenting challenges for test development and commercialization. The level of multiplexing in highly accurate antigen detection in LFT formats remains limited to just a few targets (in general, less than five, and often not more than two).
Cost-effective decentralized testing
LFTs need to be integrated within a surveillance system or a care pathway, alongside other preventative, therapeutic and diagnostic tools. LFT research has long focused on early-stage technologies; however, real-world use should be investigated, including individual, setting and system-level design considerations to ensure that end-users are linked to care86 and that test results inform surveillance and infection-control interventions. Digital care pathways can link LFT self-tests to health systems and electronic patient records (Fig. 4), as was demonstrated by digitally linking self-sampling for chlamydia to care in a proof-of-concept online pathway in the UK87. Similarly, digital tools have been integrated with community-based testing using LFTs in South Africa, increasing case detection, reporting and follow-up88.
Patients should be encouraged to report their results so that they can be linked to care and advice through digital capture; in parallel, digital tools should be designed to ease the burden on patients, and improve provider-to-provider communication. Although control of test results may be advantageous for privacy reasons, care-seeking and behavioural changes also occur without digitally reporting positive test results. Although the importance of reporting varies by pathogen and setting, self-testing and control over the disclosure of results are a key benefit in making diagnostics accessible, as has been shown in demographics hesitant to test for HIV in traditional clinic settings78. Importantly, self-testing and digital reporting have shown perceived privacy benefits compared to in-person testing89.
New LFTs are needed for the diagnosis of various infections, such as urine-based tuberculosis testing, neglected tropical diseases testing90, LFTs to support the triple elimination of mother-to-child transmission of HIV, syphilis and HBV, and improvements to malaria LFTs to ensure full coverage of pathogenic species and genetic evolution in the parasites.
Implementation research or randomized controlled trials can identify the effectiveness and cost effectiveness of LFT strategies, including test-and-treat programmes linking high-risk people to antivirals (such as nirmatrelvir/ritonavir, Paxlovid)91 for SARS-CoV-2. Similar approaches can inform the use of therapies for other infections, such as respiratory syncytial virus, and of currently underused therapies, such as oseltamivir for influenza.
LFTs can also be used for monitoring chronic infections and response to treatment. For example, future LFTs capable of viral load monitoring could empower people with HIV to self-monitor, as do glucose tests for people with diabetes. Nucleic-acid-based LFTs may also be amenable to conditions such as human papillomavirus (HPV)-linked cervical cancer. Beyond human health, LFTs could find application in animal health and environmental monitoring92, for example, in wastewater-based epidemiology.
Next-generation lateral flow tests
Bioengineering approaches can aid in improving the sensitivity, specificity, sample collection and digital data capture of LFTs.
Sensitivity and specificity
Increasing the sensitivity of LFTs could democratize decentralized testing. The sensitivity of LFTs is limited mainly by the nanoparticle properties, read-out methods93, binding kinetics and mass transport15. The type (for example, fluorescent or plasmonic nanoparticle) and properties (for example, size and morphology94) of nanoparticles and the corresponding read-out determine the smallest detectable number of bound nanoparticles at the test line. Assuming perfect analyte-to-nanoparticle binding, the number of bound nanoparticles translates to analyte concentration, because at the detection limit, the number of analyte molecules is smaller than the number of nanoparticles, assuming approximately one analyte molecule per bound particle. To optimize performance, the ratio of the signal provided by each nanoparticle to the background signal produced by substrates, samples or the environment needs to be maximized. In reality, however, binding is imperfect and described by receptor–ligand kinetics and mass transport, which determine specific (analyte-mediated binding) and non-specific binding rates. Therefore, the ratio of the signal provided by each specifically bound nanoparticle to the signal produced by non-specifically bound particles and the background arising from substrates, samples or the environment needs to be optimized.
Sensitivity is typically reduced by low-signal positive samples near the detection limit, whereas specificity is decreased by negative samples with high signal. Therefore, sensitivity can be improved by lowering specificity and vice versa, affecting interrelated assay design choices, such as nanoparticle concentration and the surface density of capture ligands; here, higher concentrations of nanoparticles can increase specific binding rates at low analyte concentrations, but can also increase non-specific binding (depending on nanoparticle properties and surface chemistry). Decreasing the flow rate similarly increases specific and non-specific binding. Reducing non-specific binding by optimizing buffers allows higher nanoparticle concentrations without compromising specificity. Optimization, however, is limited by sample type, test time and ease of use, for example, the lack of a centrifuge at the point of care. The choice of materials95 and architecture96 of LFTs — membrane, conjugate, sample and absorbent pads, blocking materials and buffers — all determine the playoff between specific and non-specific interactions; for example, smaller pore-size membranes can have a higher sensitivity per volume of sample, at the cost of slower flow rates. In addition, complex samples, such as faeces or whole blood, may require processing and extraction steps.
Theory and modelling approaches can also be applied to study the mechanisms underlying test sensitivity and specificity, for example, by integrating reaction and mass transport theory to generate computational models15. Sensitivity may be further improved by bottom-up, target-focused approaches, using high-affinity receptors and amplification strategies, for example, and top-down device engineering approaches to improve the signal-to-noise ratio of transducers and nanoparticle readout.
Sample collection and preparation
The quality of tests and samples affects sensitivity and specificity97. Sample collection and preparation are essential steps in any assay, but are often not addressed in the academic literature, and are not well adapted to the setting in which the test is administered. Samples tested on LFTs — including from whole blood, plasma, serum, saliva, urine, stool, vaginal, sputum, nasal and nasopharyngeal swabs — have diverse properties and compositions11, which need to be considered in the test design. Integrating blood lancets98 can reduce the number of components and handling of sharps. LFT sample pads and buffers can even the flow, control sample buffering11 or act as a filter; however, some specimens (such as serum) require pre-treatment after sampling.
Sample preparation typically includes extraction, purification and concentration, which must be accomplished without sacrificing usability or reproducibility. Protocols for LFT sample preparation depend on sample type, assay, target and setting, and can be optimized for high-throughput, rapid or point-of-care testing99. For example, paramagnetic particle systems can be used for purification and concentration to enable automated, high-throughput testing100, and magnetic-bead-based commercial kits allow rapid, point-of-care sample preparation101. Magnetic nanoparticles can also be harnessed for sample enrichment and detection in the same LFT102.
Molecular testing, in particular RT-PCR, is typically less robust against contaminants and inhibitors103 than immunoassays. Alternatively, nucleic acid amplification tests, often based on isothermal amplification (for example, recombinase polymerase amplification (RPA) or loop-mediated isothermal amplification (LAMP)), can be integrated with an LFT read-out14,104, moving nucleic acid amplification tests closer to point-of-care testing.
Sensitive nucleic acid detection
The combination of LFT test formats with nucleic acid amplification and detection heralds a new era of highly sensitive and specific infectious disease diagnostics105,106,107,108,109,110. A number of products are in development, and at least one company has achieved FDA approval, integrating standard RT-PCR amplification and an LFT readout into an easy-to-use format. A single-use disposable molecular test is also commercially available for COVID-19 and sexually transmitted infection testing from self-collected swabs10. Isothermal LAMP or RPA amplification can be combined with LFT outputs using functionalized primers to create dual hapten-labelled amplicons that bind to both the test strip and a colorimetric label. These methods are often highly sensitive; however, non-specific amplification can result in decreased specificity, and there can be compatibility issues of the amplification formulation with LFT test line binding. Amplification for LFT nucleic acid detection can also be achieved by displacement amplification or rolling circle amplification111.
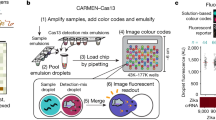
Cited as a ‘technology to watch out for’ in 2022 (ref. 112), CRISPR-based diagnostic (CRISPR-Dx) systems, such as specific high-sensitivity enzymatic reporter unlocking (SHERLOCK)113,114 and DNA endonuclease-targeted CRISPR trans reporter (DETECTR)115, increase the range of molecular targets suitable for LFT-based detection and have been used for diagnosis of SARS-CoV-2116,117 (Fig. 5a). Typically, isothermal molecular amplification, such as reverse transcription (RT)-LAMP or RT-RPA, is used as an initial step to improve diagnostic sensitivity, followed by a CRISPR-based detection step triggered through highly specific recognition of a target nucleic acid sequence by a guiding RNA (gRNA)–Cas complex. Here, Cas12 or Cas13 are mostly used, which collaterally cleave a reporter when activated by target binding. Once cleaved by the activated Cas, the reporter can bind to the LFT test line as well as to the control line. Alternatively, dead Cas9 (dCas9) binds target sequences without cutting, resulting in co-localization of the dCas9, target DNA, and a nanoparticle-based colorimetric label at the LFT test line.
a, Cas-based reactions can be combined with a nanozyme-amplified lateral flow test (LFT). Target RNA is mixed with the guiding RNA (gRNA)–Cas13 complex and reporter RNA to trigger the clustered regularly interspaced short palindromic repeats (CRISPR) reaction. Subsequently, streptavidin-functionalized nanozymes are mixed with the CRISPR reaction product that contains the biotinylated reporter RNA to form a complex. The test strip is preprinted with anti-fluorescein amidite to draw up the mixture. The uncleaved reporter RNA–nanozyme complexes are captured at the test line. Finally, the substrate is added for colour development. b, Spin-enhanced quantum nanodiamond sensing and background subtraction can be implemented in LFTs to enable ultra-sensitive virus detection. The scanning electron micrographs show nanodiamonds. Pixel intensity variation is shown at the test line on an LFT strip with immobilized nanodiamonds under an amplitude-modulated microwave field. Background subtraction allows ultra-sensitive virus detection. In the amplitude-modulated field, mean fluorescence intensity varies over time. A lock-in algorithm quantifying modulation amplitude over a range of frequencies, gives a sinc function with a peak at the modulation frequency. Nanodiamonds are immobilized at the test line in a sandwich structure in the presence of double-stranded DNA (dsDNA) amplicons. AU, arbitrary units. c, Healthcare workers collect images of human immunodeficiency virus (HIV) LFTs in the field, and machine learning allows automatic classification of LFT results. d, Deep-learning-enabled point-of-care sensing using multiplex paper-based sensors and a mobile-phone reader with an inserted vertical flow assay cassette. The algorithmically determined immunoreaction spot layout of the multiplexed vertical flow assay membrane contains several distinct spotting conditions, each of which uniquely reacts with the sensed analyte and the signal-forming gold nanoparticles. CN/DAB; 4-chloro-1-naphthol/3,3′-diaminobenzidine, tetrahydrochloride. Part a adapted from ref. 131, Springer Nature Limited. Part b adapted with permission from ref. 138, Elsevier, adapted from ref. 13, Springer Nature Limited, and reprinted from ref. 13, Springer Nature Limited. Part c image courtesy of African Health Research Institute. Part d adapted from ref. 165, Springer Nature Limited.
CRISPR-Dx are versatile platforms, ideal for rapid outbreak response, with the first laboratory CRISPR-Dx for COVID-19 available within months of the beginning of the pandemic118. Importantly, CRISPR-Dx can be more sensitive than antigen LFTs for COVID detection119,120, and integrate multi-step and thus, more streamlined protocols, moving towards translation from the laboratory to the point of care and resource-limited settings. The (SHERLOCK testing in one pot) STOPCovid121, COVID SHINE (SHERLOCK and HUDSON Integration to Navigate Epidemics)120,122 and a wearable COVID-19 face mask123 demonstrate highly sensitive LFT detection with streamlined protocols, involving only one or two user interactions. Several assays124,125,126 have focused on developing COVID-19 LFT diagnostic protocols using minimum equipment with the potential to be portable. Finally, driven by the need to identify COVID variants, CRISPR has also been used to detect mutations, including single nucleotide variations119,120,127.
CRISPR-Dx could extend beyond COVID-19 LFT diagnosis and may allow rapid diagnosis of diverse diseases and variant or resistance monitoring. In particular, CRISPR-Dx benefit from high sensitivity in diverse clinical samples110,119,128,129, streamlined ‘one-pot’ protocols and freeze-dried, cell-free assay formats for usability and stability121,122,123,127,130,131, as well as smartphone-integrated result interpretation contributing to digital surveillance programmes122,127,132. CRISPR-Dx have initially required laboratory equipment for amplification and readout; however, these platforms can also operate with battery power or without power at room temperature123,125,127. For antimicrobial resistance monitoring, however, isothermal molecular amplification131 needs to be avoided, and multiplexing should be implemented to improve accuracy, sensitivity and variant detection114,119,120,126,127,132,133. Importantly, CRISPR-Dx could be applied in low-resource settings, which will require integration into commercially viable products and field evaluations.
Materials and sensors
Sensitivity and specificity can be improved by developing receptor ligands with high kinetic on-rates and low non-specific binding, such as nanobodies134,135. Owing to their small size (around 15 kDa), they can reach less accessible paratopes of analyte molecules and confer greater chemical and thermal stability than antibodies. Complementary to improving binding at low-analyte concentrations, methods are being developed to multiply the number of targets.
‘Top-down’ amplification strategies can improve detection limits. In most LFTs, optical absorption-based imaging of plasmonic nanoparticles determines the detection limit, governed by the lowest number of particles required to produce a detectable change in light absorption and the number of binding events required to reach that threshold. Size optimization of gold nanoparticles93, the design of catalytical nanoparticles that develop a chromogenic substrate136 and other chemical modifications137 can further increase the signal per particle. In addition, read-out methods can be improved to reduce the number of required binding events; for example, the plasmonic peak can be used to subtract background in two-wavelength imaging138, or alternative read-outs can be applied, such as thermal contrast139.
Dual dynamic range regimes136, signal amplification strategies140, sensitive labels141, and dual-wavelength-based imaging138 have improved the analytical sensitivity and dynamic range of paper-based biosensors, improving their quantification capabilities. Fluorescent nanoparticles, such as quantum dots142,143, have also been investigated to improve detection limits. Similarly, sensitivity is limited by the signal-to-noise ratio, that is, the absolute signal can be increased by longer exposure times; however, background and sample autofluorescence mask low signals. Nanoparticle signals can be separated from background fluorescence by spin manipulation of fluorescent nanodiamonds13 (Fig. 5b). However, fluorescence-based imaging approaches require a dedicated reader, adding cost and complexity. Alternatively, mobile-phone-based144 readers, standalone readers15 and optics integrated into the test cassette68,145 have been explored. Surface-enhanced Raman scattering (also requiring a reader) may enable sensitive readout146,147,148, and separate detection of specific and non-specific binding149 (Fig. 5c). In addition, magnetic150 and electrochemical151 transduction techniques have been demonstrated for LFTs.
Signal enhancement strategies are limited by non-specific binding, because lowering the detection limits increases the detection of non-specific binding. Designing the transduction mechanism to differentiate between specifically and non-specifically bound labels, by producing a signal only for particles bound to capture ligands152 could improve sensitivity, allowing high nanoparticle concentrations and thus, rapid binding kinetics without increasing negative signals.
LFTs using fluorescent nanoparticles or other labels that require specific excitation conditions often need additional hardware for automated result capture (a ‘reader’). Here, sensitivity and stability of measurement should outperform stand-alone LFTs153,154,155,156. Such readers can be used to detect approximately 80 fluorophores per diffraction-limited spot size, potentially pushing the limit of sensitivity to the single-molecule level157,158. This might not be appropriate in some applications, such as self-testing, for which additional components reduce affordability and usability. However, LFT–reader combinations could be economical in the clinic and for use by healthcare workers, where multiple tests are carried out by a single user. Here, portability, speed and affordability will probably compare positively with laboratory testing. Although requiring specialist hardware, digital connectivity or readers using smartphone cameras could automate result capture. However, readers can limit the volume of tests that can be performed, and some commercially available readers are only guaranteed by manufacturers for a limited number of tests before replacement. More compact readers, zero maintenance and cost-effectiveness159 could address this bottleneck.
Multiplexing
Decentralized testing using multiplex LFTs (xLFTs) with data capture and reporting may provide early alerts of outbreaks, help to detect infections and antimicrobial resistance, and support effective triaging in health systems. For example, xLFTs can detect multiple targets and differentiate between multiple flaviviruses160, sexually transmitted infections and drugs. xLFTs have also been developed to distinguish SARS-CoV-2, influenza and their co-infections161, and HIV/syphilis xLFTs162 are commercially available. xLFTs combined with symptom and demographic data could pave the way for nuanced decision-making when paired with digital tools; however, systemic and engineering challenges (such as cross-reactivity or interference between multiple test lines in the limited test strip area, reducing specificity) have limited xLFT commercialization thus far. In addition, the need to identify a single set of LFT parameters (for example, buffer and materials) for each target analyte compromises sensitivity.
Cross-reactivity can be mitigated by implementing multiple parallel flow pathways, vertical flow assays163, or other paper-based configurations164 with spatial separation of the immunoreaction spots and perpendicular flow of the sample fluid through the membrane. Vertical flow assays can contain about 100 spatially isolated immunoreaction spots in a single test165 (Fig. 5d), and contain the same assay reagents and inexpensive materials often used in LFTs, enabling manufacturing scale-up. However, vertical assays may result in lower sensitivity owing to the short binding time.
Large-scale manufacturing of xLFTs requires the printing of multiple test spots in a single disposable strip, and thus, multiple dispensing nozzles with different quality-control measures, potentially increasing production costs. Furthermore, additional test lines complicate result interpretation, although this can be mitigated through the use of digital interpretation or test spot array patterns that are recognizable to users166,167. The development and commercialization of xLFTs are further limited by the need to validate multiple biomarkers with clinical importance, low market demand and a complicated regulatory approval pathway.
Digital connection and deep learning
The future of public health is increasingly digital. As of 2019, 65% of the global population subscribe to mobile phones, with the fastest growth in sub-Saharan Africa168. Accordingly, large-scale, real-world image datasets could be used to train and validate image classification models19,71,169 (Fig. 5c) for digital LFT data capture, including in low- and middle-income regions19. These datasets can be expensive to produce, and the number of real-world positive cases may vary by disease and setting. However, commercial test providers and public health agencies are already collecting images for test registration and verification, and thus, image collection could be automated in these pipelines to continually improve image classification models. Test providers may be hesitant to introduce algorithms requiring updates when new tests are deployed; however, given the similar visual appearance of qualitative LFT results, algorithms could be updated with smaller datasets once a large dataset is captured for a single test. Test registration and result entry with a single photograph can reduce the data entry burden and encourage users to report results. Probabilistic algorithms providing a measure of uncertainty can reduce confusion arising from false results for users170.
Smartphone-read results allow more complex LFT configurations, including quantitative and multiplex tests, without increasing complexity for the user. In addition, information can be linked to each measurement, enabling real-time, geo-linked surveillance154. Digital solutions should prioritize interoperability and integrate with existing platforms (not exacerbate digital exclusion), should encourage trust with easy-to-use systems and protect personal information, and they should be co-designed with end-users for optimized usability89. Moreover, machine learning can be applied to optimize, analyse and quantify paper-based multiplexed tests165. However, widespread use of transformative digital solutions will need to be implemented in existing healthcare pathways; these solutions will require acceptability, high data quality and access; and legal, ethical, privacy and data security, and organizational and workforce barriers must be overcome66.
Green manufacturing
LFTs are typically single use and disposable, producing non-biodegradable plastic waste171 and sometimes also electronic waste. As an alternative to plastic components, card or biodegradable plastics have been implemented in commercially available tests172. However, it is important that these materials retain the advantages of plastic, including robustness and protection of the assay strip, the ability to create the pressure points necessary for controlled flow and low-cost and scalable manufacturing methods. In addition, they should be lightweight, easily transportable and easily printable to include QR codes and lot numbers. In clinical settings, LFTs may often need to be incinerated regardless of material composition, although materials could be included that reduce the environmental toxicity of LFT components and the volume of packaging.
The environmental impact of LFTs that contain new materials and components needs to be considered. The REASSURED guidelines9 assert the need for environmentally friendly tests that do not require non-existent waste infrastructure or risk the introduction of toxic chemicals into the environment9. For example, synthetic biology can be used for animal-free antibody production173, reducing the overall carbon footprint. If electronic components are necessary, paper-based batteries174 may aid in reducing toxic waste. Moreover, multiplexed tests can reduce the use of multiple tests.
The sustainability of LFTs can be improved by frequent evaluation of the usage of tests in domestic settings by manufacturers. In addition, redundant components and the size of components could be reduced, or components could be combined. Regulators can improve LFT sustainability by introducing incentives for reduction of materials and toxic chemicals, sustainable design and reusable components, by introducing regulation for the clear labelling of recyclable components, flexible to different disposal requirements for different modes of disease transmission, and by reducing regulatory barriers to changes in packaging and cassette design.
Translation
The LFT and broader in vitro diagnostics market have historically had smaller investments owing to high technological and regulatory barriers, a perceived low health economic value, and smaller financial returns compared to vaccines and therapeutics. Additional barriers, such as unpredictable demand, as well as manufacturing and distribution challenges, further limit the scale-up of new diagnostics.
Target product profiles, technical specifications series175 and preferred product characteristics176 are strategic documents that can be used by manufacturers to guide the fast-tracked development of products, and to assist in the identification of regulatory requirements, based on use cases. In autumn 2020, the WHO published four priority target product profiles for COVID-19 diagnostics46.
Manufacturers should use information gained from the implementation of device risk management and the development of a regulatory strategy driven by implementation and impact requirements. The regulatory strategy should not only consider regulatory requirements in the proposed regions of sale, but also assay quality assurance measures of global buyers, to determine whether further testing or additional quality assurance steps are required.
LFTs and accompanying testing kits and processes need to be designed to be inclusive and easy to use by people with diverse health literacy in various settings177. This extends to guidelines for delivery, sample pack design and user instructions178, minimally invasive biological sampling, and a minimum number of sample preparation steps9.
Tests are often developed and validated using synthetic samples, which may mask challenges introduced by real-world samples, such as non-specific binding. Access to qualified specimens as well as reference and control materials (ideally by WHO international standards) are key to assay validation (including clinical evaluation). In particular, bio-banked, characterized, clinical specimens assist in the development, verification, validation and quality assurance of assays. In addition, availability of reference laboratories, access to reference methods and technology-appropriate written standards (ideally international in nature) are important.
Only a few proof-of-concept diagnostics technologies have been translated into commercially available products. To increase translation, the ultimate product requirements need to be considered early in the research process, not just in terms of target analytical and clinical performance, but also regarding robustness (including stability in temperature and humidity, and over time), time to test results, ease of use, connectivity and affordability. Moreover, manufacturability and scale-up need to be ensured.
Research often focuses on specific elements of diagnostic tests, which may be broadly applicable and disease-agnostic; however, each diagnostic product is intended for a specific clinical application, target user, testing population and use setting. Product design should thus meet the requirements for each specific application and consider end-user needs. Co-creation, community engagement and gold standard frameworks for evaluation are needed for effective test deployment, including early engagement of end-users, healthcare providers, academic researchers, industry, public health authorities and public health agencies.
Outlook
LFTs have been hailed “the heroes of the pandemic”179, transforming COVID-19 testing globally. This simple, low-cost platform is gaining the recognition it deserves, but has long been underfunded, overshadowed by investments in laboratory-based and point-of-care molecular and sequencing diagnostics. Moreover, there are major inequities in access to tests, raising ethical concerns and affecting our collective ability to respond to the pandemic.
Bioengineering will play a key part in increasing the sensitivity and specificity of LFTs, enabling multiplexing and data capture, as well as manufacturing in low-resource settings180. The combination of LFT test formats with nucleic acid amplification and detection could provide the next generation of LFTs, albeit currently limited to one product on the market. Moreover, emerging technologies could be implemented in LFTs, such as the use of nano- and quantum materials to improve sensitivity, CRISPR to improve specificity and deep learning approaches to allow digital connectivity and quality assurance.
However, a reduction in funding for LFT research post COVID-19 may hamper efforts to capitalize on gains in decentralized testing, especially self-testing, which may be critical to address future pandemic threats. Prior to COVID-19, funding for infectious disease research declined between 2007 and 2018 (ref. 74). In 2021, the UK reduced its commitment to overseas development aid from 0.7% to 0.5% of GDP, with some research funding cut by up to 85% (ref. 181). However, coordinated long-term investment in a global network of R&D LFT hubs is needed to develop and retain people and skillsets, to share knowhow, to standardize reagents and to pilot ‘test beds’ in which to evaluate effectiveness and cost-effectiveness, and to grow the manufacturing capability developed during the pandemic. These hubs could help to nurture a pipeline of innovative bioengineering approaches across the translational ‘valley of death’.
COVID-19 typically presents high viral load (and antigen levels), which can be detected by currently available LFTs; however, other diseases may prove more challenging to detect using LFTs. Neither LFTs nor point-of-care tests currently exist for 50% of the WHO priority diseases of epidemic potential. Importantly, LFTs are also urgently needed to detect antimicrobial resistance, human papillomavirus-associated cervical cancers, and acute and chronic infections, as well as for viral load and animal and environmental monitoring. Globally convergent regulatory pathways are needed, tackling issues of intellectual property, expanding generic (non-branded) diagnostic production capability in low- and middle-income regions to bring down costs182, and making tests manufacturable, accessible, acceptable and usable by the broadest cross-section of society. For LFTs to be successful in reducing transmission and linking patients to care, investment in communication and education on their use is needed, updated according to continual monitoring of testing behaviours. Linking to care pathways, harnessing digital technologies66 wherever feasible and acceptable, and co-creation of tests with end-users are essential168.
In the wake of the COVID-19 pandemic, it is time for governments around the world to embrace some ‘lateral thinking’ and dare to dream the future of decentralized health and affordable self-testing. Bioengineering and LFTs will play a key part in democratizing health, ensuring we honour the Sustainable Development Goals and “leave no one behind”183, and strengthening resilience before the next pathogen strikes.
References
The ACT-Accelerator: two years of impact. WHO https://www.who.int/publications/m/item/the-act-accelerator--two-years-of-impact (2022).
Mina, M. J., Parker, R. & Larremore, D. B. Rethinking Covid-19 test sensitivity — a strategy for containment. N. Engl. J. Med. 383, e120 (2020). This perspective examines the use of SARS-CoV-2 rapid antigen tests as a strategy for containment.
Singer, J. M. & Plotz, C. M. The latex fixation test. I. Application to the serologic diagnosis of rheumatoid arthritis. Am. J. Med. 21, 888–892 (1956).
Rosenstein, R. W. & Bloomster, T. G. Solid phase assay employing capillary flow. US patent US-4855240-A (1987).
Charlton, D. E. Test device and method for colored particle immunoassay. US patent US21158288A (1988).
WHO recommends HIV self-testing — evidence update and considerations for success. WHO https://www.who.int/publications-detail-redirect/WHO-CDS-HIV-19.36 (2019).
Use of SARS-CoV-2 antigen-detection rapid diagnostic tests for COVID-19 self-testing. WHO https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Ag-RDTs-Self_testing-2022.1 (2022). World Health Organization interim guidance on recommending COVID-19 self-testing using SARS-CoV-2 antigen tests; the web annexes include useful information on implementation.
Peeling, R. W., Heymann, D. L., Teo, Y.-Y. & Garcia, P. J. Diagnostics for COVID-19: moving from pandemic response to control. Lancet https://doi.org/10.1016/S0140-6736(21)02346-1 (2021).
Land, K. J., Boeras, D. I., Chen, X.-S., Ramsay, A. R. & Peeling, R. W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 4, 46–54 (2019). This article reports the go-to criteria for developing field-ready rapid diagnostics.
Visby MedicalTM receives FDA clearance and CLIA waiver at the point of care for PCR sexual health test. Visby Medical https://www.visbymedical.com/news/visby-medical-receives-fda-clearance-and-clia-waiver-at-the-point-of-care-for-pcr-sexual-health-test/ (2021).
Parolo, C. et al. Tutorial: design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 15, 3788–3816 (2020). This protocol outlines the development of a lateral flow test.
Huang, X., Jain, P. K., El-Sayed, I. H. & El-Sayed, M. A. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2, 681–693 (2007).
Miller, B. S. et al. Spin-enhanced nanodiamond biosensing for ultrasensitive diagnostics. Nature 587, 588–593 (2020). This article reports how the quantum properties of nanodiamonds can be exploited to develop ultrasensitive lateral flow tests.
Liu, Y., Zhan, L., Qin, Z., Sackrison, J. & Bischof, J. C. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 15, 3593–3611 (2021).
Gasperino, D., Baughman, T., Hsieh, H. V., Bell, D. & Weigl, B. H. Improving lateral flow assay performance using computational modeling. Annu. Rev. Anal. Chem. 11, 219–244 (2018). This review discusses the improvement of lateral flow test assay performance using computational modelling.
Fu, E., Ramsey, S. A., Kauffman, P., Lutz, B. & Yager, P. Transport in two-dimensional paper networks. Microfluid. Nanofluid. 10, 29–35 (2011).
Rapid lateral flow test strips: considerations for product development. Merck Millipore https://www.merckmillipore.com/INTERSHOP/web/WFS/Merck-RU-Site/ru_RU/-/USD/ShowDocument-Pronet?id=201306.15671 (2013).
Mukadi, P. et al. External quality assessment of reading and interpretation of malaria rapid diagnostic tests among 1849 end-users in the Democratic Republic of the Congo through short message service (SMS). PLoS One 8, e71442 (2013).
Turbé, V. et al. Deep learning of HIV field-based rapid tests. Nat. Med. 27, 1165–1170 (2021). This article reports the collection of a large image dataset of real-world HIV lateral flow tests by healthcare workers in South Africa to develop accurate lateral flow test image classification models.
Vandenberg, O., Martiny, D., Rochas, O., van Belkum, A. & Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 19, 171–183 (2021).
Antigen-detection in the diagnosis of SARS-CoV-2 infection. WHO https://www.who.int/publications-detail-redirect/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (2021).
Merrick, B. et al. Real-world deployment of lateral flow SARS-CoV-2 antigen detection in the emergency department to provide rapid, accurate and safe diagnosis of COVID-19. Infect. Prev. Pract. 3, 100186 (2021).
Reynard, C. et al. COVID-19 rapid diagnostics: practice review. Emerg. Med. J. 39, 70–76 (2022).
Garciá-Fiñana, M. et al. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: population based cohort study. Br. Med. J. 374, 1637 (2021).
Holt, E. COVID-19 testing in Slovakia. Lancet Infect. Dis. 21, P32 (2021).
England Summary | Coronavirus (COVID-19) in the UK. Gov.uk https://coronavirus.data.gov.uk (2022).
Davies, B. et al. Acceptability, usability, and performance of lateral flow immunoassay tests for severe acute respiratory syndrome coronavirus 2 antibodies: REACT-2 study of self-testing in nonhealthcare key workers. Open Forum Infect. Dis. 8, ofab496 (2021).
Goggolidou, P., Hodges-Mameletzis, I., Purewal, S., Karakoula, A. & Warr, T. Self-testing as an invaluable tool in fighting the COVID-19 pandemic. J. Prim. Care Commun. Health 12, 21501327211047784 (2021).
Wanat, M. et al. Perceptions on undertaking regular asymptomatic self-testing for COVID-19 using lateral flow tests: a qualitative study of university students and staff. BMJ Open 11, e053850 (2021).
Møller, I. J. B., Utke, A. R., Rysgaard, U. K., Østergaard, L. J. & Jespersen, S. Diagnostic performance, user acceptability, and safety of unsupervised SARS-CoV-2 rapid antigen-detecting tests performed at home. Int. J. Infect. Dis. 116, 358–364 (2022).
Cassuto, N. G. et al. Evaluation of a SARS-CoV-2 antigen-detecting rapid diagnostic test as a self-test: diagnostic performance and usability. J. Med. Virol. 93, 6686–6692 (2021).
Tonen-Wolyec, S. et al. Evaluation of the practicability of BIOSYNEX Antigen Self-Test COVID-19 Ag+ for the detection of SARS-CoV-2 nucleocapsid protein from self-collected nasal mid-turbinate secretions in the general public in France. Diagnostics 11, 2217 (2021).
Lindner, A. K. et al. Diagnostic accuracy and feasibility of patient self-testing with a SARS-CoV-2 antigen-detecting rapid test. J. Clin. Virol. 141, 104874 (2021).
Iruzubieta, P. et al. Feasibility of large-scale population testing for SARS-CoV-2 detection by self-testing at home. Sci. Rep. 11, 9819 (2021).
Shilton, S., Ivanova Reipold, E., Roca Álvarez, A. & Martínez-Pérez, G. Z. Assessing values and preferences toward SARS-CoV-2 self-testing among the general population and their representatives, health care personnel, and decision-makers: protocol for a multicountry mixed methods study. JMIR Res. Protoc. 10, e33088 (2021).
Thomas, C. et al. Values and preferences of the general population in Indonesia in relation to rapid COVID-19 antigen self-tests: a cross-sectional survey. Trop. Med. Int. Health 27, 522–536 (2022).
Ingold, H. et al. The Self-Testing AfRica (STAR) initiative: accelerating global access and scale-up of HIV self-testing. J. Int. AIDS Soc. 22, e25249 (2019).
Emergency use listing procedure. WHO https://www.who.int/publications/m/item/emergency-use-listing-procedure (2020).
Pickering, S. et al. Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: a single-centre laboratory evaluation study. Lancet Microbe 2, e461–e471 (2021).
Killingley, B. et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 28, 1031–1041 (2022).
Dinnes, J. et al. Rapid, point‐of‐care antigen tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD013705.pub2 (2021).
Larremore, D. B. et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci. Adv. 7, eabd5393 (2021).
Peto, T. et al. COVID-19: rapid antigen detection for SARS-CoV-2 by lateral flow assay: a national systematic evaluation of sensitivity and specificity for mass-testing. eClinicalMedicine 36, 100924 (2021).
Cevik, M. et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2, e13–e22 (2021).
Peeling, R. W., Olliaro, P. L., Boeras, D. I. & Fongwen, N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect. Dis. 21, e290–e295 (2021).
COVID-19 target product profiles for priority diagnostics to support response to the COVID-19 pandemic v.1.0. WHO https://www.who.int/publications/m/item/covid-19-target-product-profiles-for-priority-diagnostics-to-support-response-to-the-covid-19-pandemic-v.0.1 (2020).
FIND evaluation of SARS-CoV-2 antigen (Ag) detecting tests. FIND https://www.finddx.org/sarscov2-eval-antigen/ (2022).
Bekliz, M. et al. Analytical performance of eleven SARS-CoV-2 antigen-detecting rapid tests for Delta variant. Preprint at medRXiv https://doi.org/10.1101/2021.10.06.21264535 (2021).
Bekliz, M. et al. Sensitivity of SARS-CoV-2 antigen-detecting rapid tests for Omicron variant. Microbiol. Spectr.10, e00853-22 (2022).
Scheiblauer, H. et al. Comparative sensitivity evaluation for 122 CE-marked rapid diagnostic tests for SARS-CoV-2 antigen, Germany, September 2020 to April 2021. Euro Surveill. 26, 2100441 (2021).
Garciá-Finaña, M. & Buchan, I. E. Rapid antigen testing in covid-19 responses. Science 372, 571–572 (2021).
SARS-CoV-2 lateral flow antigen tests: evaluation of VOC1 (Kent, UK) and VOC2 (South Africa). Gov.uk https://www.gov.uk/government/publications/sars-cov-2-lateral-flow-antigen-tests-evaluation-of-voc1-and-voc2/sars-cov-2-lateral-flow-antigen-tests-evaluation-of-voc1-kent-uk-and-voc2-south-africa (2021).
Bekliz, M. et al. SARS-CoV-2 antigen-detecting rapid tests for the Delta variant. Lancet Microbe 3, e90 (2022).
Raïch-Regué, D. et al. Performance of SARS-CoV-2 antigen-detecting rapid diagnostic tests for Omicron and other variants of concern. Front. Microbiol. 13, 810576 (2022).
RADx programs. National Institutes of Health (NIH) https://www.nih.gov/research-training/medical-research-initiatives/radx/radx-programs (2020).
Measuring the economic output of COVID-19 testing, tracing and vaccinations: April 2020 to June 2021. ONS Gov.uk https://www.ons.gov.uk/economy/grossdomesticproductgdp/methodologies/measuringtheeconomicoutputofcovid19testingtracingandvaccinationsapril2020tojune2021#measurement-of-covid-19-testing-tracing-and-vaccination-services-prior-to-the-april-to-june-2021-quarterly-national-accounts (2021).
Fleming, K. A. et al. The Lancet Commission on diagnostics: transforming access to diagnostics. Lancet 398, 1997–2050 (2021).
BinaxNOWTM COVID-19 Ag card. FDA https://www.fda.gov/media/141570/download (2020).
Foundation for innovative new diagnostics. The Rockfeller Foundation https://www.rockefellerfoundation.org/grant/grant-2020-378/ (2022).
100 days mission to respond to future pandemic threats. Gov.uk https://www.gov.uk/government/publications/100-days-mission-to-respond-to-future-pandemic-threats (2021).
Nkengasong, J. Let Africa into the market for COVID-19 diagnostics. Nature 580, 565–565 (2020).
Hannay, E., Fernández-Suárez, M. & Duneton, P. COVID-19 diagnostics: preserving manufacturing capacity for future pandemics. BMJ Glob. Health 7, e007494 (2022).
Bright, B. et al. COVID-19 preparedness: capacity to manufacture vaccines, therapeutics and diagnostics in sub-Saharan Africa. Global. Health 17, 24 (2021).
Local diagnostics to meet local health needs. Médecins Sans Frontières https://msfaccess.org/improve-local-production-diagnostics (2021).
UK House of Commons Committee of Public Accounts. Test and trace update. UK Parliament https://publications.parliament.uk/pa/cm5802/cmselect/cmpubacc/182/summary.html (2021).
Budd, J. et al. Digital technologies in the public-health response to COVID-19. Nat. Med. 26, 1183–1192 (2020).
Recommendations on digital interventions for health system strengthening. WHO https://www.who.int/publications-detail-redirect/9789241550505 (2019).
FDA authorizes Ellume COVID-19 home test as first over-the-counter fully at-home diagnostic test. Ellume https://www.ellumehealth.com/2020/12/15/fda-authorizes-ellume-covid-19-home-test-as-first-over-the-counter-fully-at-home-diagnostic-test/ (2020).
Park Chunjong et al. The design and evaluation of a mobile system for rapid diagnostic test interpretation. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. https://doi.org/10.1145/3448106 (2021).
Haisma, S.-M. et al. Head-to-head comparison of three stool calprotectin tests for home use. PLoS One 14, e0214751 (2019).
Wong, N. et al. Machine learning to support visual auditing of home-based lateral flow immunoassay self-test results for SARS-CoV-2 antibodies. Commun. Med. 2, 78 (2022).
Banathy, R. et al. Machine learning for determining lateral flow device results in asymptomatic population: a diagnostic accuracy study. Preprint at https://doi.org/10.2139/SSRN.3861638 (2021).
Arnold, C. Home testing for syphilis gains support in wake of COVID. Nature 605, 598–599 (2022).
Head, M. G. et al. The allocation of US$105 billion in global funding from G20 countries for infectious disease research between 2000 and 2017: a content analysis of investments. Lancet Glob. Health 8, e1295–e1304 (2020).
An R&D blueprint for action to prevent epidemics. WHO https://www.who.int/publications/m/item/an-r-d-blueprint-for-action-to-prevent-epidemics (2016).
Rethinking diagnostics for pandemic readiness. Nat. Biomed. Eng. 6, 221–222 (2022).
Peeling, R. W., Boeras, D., Wilder-Smith, A., Sall, A. & Nkengasong, J. Need for sustainable biobanking networks for COVID-19 and other diseases of epidemic potential. Lancet Infect. Dis. 20, e268–e273 (2020).
Sithole, N. et al. Implementation of HIV self-testing to reach men in rural uMkhanyakude, KwaZulu-Natal, South Africa. a DO-ART trial sub study. Front. Public Health 9, 652887 (2021).
Murray, C. J. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Laxminarayan, R. The overlooked pandemic of antimicrobial resistance. Lancet 399, 606–607 (2022).
O’Neill, J. Tackling drug-resistant infections globally: final report and recommendations. APO20 https://apo.org.au/node/63983 (2016).
Getahun, H., Smith, I., Trivedi, K., Paulin, S. & Balkhy, H. H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 98, 442A (2020).
Rawson, T. M., Ming, D., Ahmad, R., Moore, L. S. P. & Holmes, A. H. Antimicrobial use, drug-resistant infections and COVID-19. Nat. Rev. Microbiol. 18, 409–410 (2020).
Global tuberculosis report 2021. WHO https://www.who.int/publications-detail-redirect/9789240037021 (2021).
Burnham, C.-A. D., Leeds, J., Nordmann, P., O’Grady, J. & Patel, J. Diagnosing antimicrobial resistance. Nat. Rev. Microbiol. 15, 697–703 (2017).
Toskin, I. et al. Call to action for health systems integration of point-of-care testing to mitigate the transmission and burden of sexually transmitted infections. Sex. Transm. Infect. 96, 342–347 (2020).
Estcourt, C. S. et al. The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health 2, e182–e190 (2017).
Majam, M. et al. Monitored implementation of COVID-19 rapid antigen screening at taxi ranks in Johannesburg, South Africa. Diagnostics 12, 402 (2022).
Shahmanesh, M. et al. O14.6 Mafrica: zenzele, a mobile-phone enabled HIV testing and linkage to care pathway for young people in rural South Africa. Sex. Transm. Infect. 95, A72 (2019).
Ending NTDs: together towards 2030. WHO https://www.who.int/teams/control-of-neglected-tropical-diseases/ending-ntds-together-towards-2030 (2021).
FIND and Unitaid invest US$ 50 million to speed lifesaving testing and treatment solutions to the COVID-19 pandemic’s frontlines. Unitaid https://unitaid.org/news-blog/find-unitaid-invest-50m-covid19-nov2021/ (2021).
Bedford, J. et al. A new twenty-first century science for effective epidemic response. Nature 575, 130–136 (2019).
Zhan, L. et al. The role of nanoparticle design in determining analytical performance of lateral flow immunoassays. Nano Lett. 17, 7207–7212 (2017).
Khlebtsov, B. N., Tumskiy, R. S., Burov, A. M., Pylaev, T. E. & Khlebtsov, N. G. Quantifying the numbers of gold nanoparticles in the test zone of lateral flow immunoassay strips. ACS Appl. Nano Mater. 2, 5020–5028 (2019).
Wong, R. & Tse, H. (eds) Lateral Flow Immunoassay (Humana Press, 2009).
Tsai, T.-T. et al. Development a stacking pad design for enhancing the sensitivity of lateral flow immunoassay. Sci. Rep. 8, 17319 (2018).
Kevadiya, B. D. et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 20, 593–605 (2021).
Lippman, S. A. et al. High acceptability and increased HIV testing frequency following introduction of HIV self-testing and network distribution among South African MSM. J. Acquir. Immune Defic. Syndr. 77, 279–287 (2018).
Nichols, Z. E. & Geddes, C. D. Sample preparation and diagnostic methods for a variety of settings: a comprehensive review. Molecules 26, 5666 (2021).
Safarik, I. & Safarikova, M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn. Res. Technol. 2, 7 (2004).
Yang, Z., Xu, G., Reboud, J., Kasprzyk-Hordern, B. & Cooper, J. M. Monitoring genetic population biomarkers for wastewater-based epidemiology. Anal. Chem. 89, 9941–9945 (2017).
Nash, M. A., Waitumbi, J. N., Hoffman, A. S., Yager, P. & Stayton, P. S. Multiplexed enrichment and detection of malarial biomarkers using a stimuli-responsive iron oxide and gold nanoparticle reagent system. ACS Nano 6, 6776–6785 (2012).
Schrader, C., Schielke, A., Ellerbroek, L. & Johne, R. PCR inhibitors — occurrence, properties and removal. J. Appl. Microbiol. 113, 1014–1026 (2012).
Park, B. H. et al. An integrated rotary microfluidic system with DNA extraction, loop-mediated isothermal amplification, and lateral flow strip based detection for point-of-care pathogen diagnostics. Biosens. Bioelectron. 91, 334–340 (2017).
Kaminski, M. M., Abudayyeh, O. O., Gootenberg, J. S., Zhang, F. & Collins, J. J. CRISPR-based diagnostics. Nat. Biomed. Eng. 5, 643–656 (2021). This Review discusses the emerging field of CRISPR-based diagnostics.
Mukama, O. et al. An ultrasensitive and specific point-of-care CRISPR/Cas12 based lateral flow biosensor for the rapid detection of nucleic acids. Biosens. Bioelectron. 159, 112143 (2020).
Singh, M., Bindal, G., Misra, C. S. & Rath, D. The era of Cas12 and Cas13 CRISPR-based disease diagnosis. Crit. Rev. Microbiol. https://doi.org/10.1080/1040841X.2021.2025041 (2022).
Nouri, R. et al. CRISPR-based detection of SARS-CoV-2: a review from sample to result. Biosens. Bioelectron. 178, 113012 (2021).
Wang, X. et al. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay. ACS Nano 14, 2497–2508 (2020).
Myhrvold, C. et al. Field-deployable viral diagnostics using CRISPR-Cas13. Science 360, 444–448 (2018).
Zheng, C. et al. Rapid developments in lateral flow immunoassay for nucleic acid detection. Analyst 146, 1514–1528 (2021).
Eisenstein, M. Seven technologies to watch in 2022. Nature 601, 658–661 (2022).
Gootenberg, J. S. et al. Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017).
Gootenberg, J. S. et al. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a and Csm6. Science 360, 439–444 (2018).
Chen, J. S. et al. CRISPR–Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439 (2018).
Patchsung, M. et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 4, 1140–1149 (2020).
Broughton, J. P. et al. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 38, 870–874 (2020).
Sherlock CRISPR SARS-CoV-2 kit. FDA https://www.fda.gov/media/137746/download (2020).
Casati, B. et al. Rapid, adaptable and sensitive Cas13-based COVID-19 diagnostics using ADESSO. Nat. Commun. 13, 3308 (2022).
Arizti-Sanz, J. et al. Simplified Cas13-based assays for the fast identification of SARS-CoV-2 and its variants. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00889-z (2022).
Joung, J. et al. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N. Engl. J. Med. 383, 1492–1494 (2020).
Arizti-Sanz, J. et al. Streamlined inactivation, amplification, and Cas13-based detection of SARS-CoV-2. Nat. Commun. 11, 5921 (2020).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. https://doi.org/10.1038/s41587-021-00950-3 (2021).
Ali, Z. et al. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 288, 198129 (2020).
Rauch, J. N. et al. A scalable, easy-to-deploy protocol for Cas13-based detection of SARS-CoV-2 genetic material. J. Clin. Microbiol. 59, e02402-20 (2021).
Ali, Z. et al. Bio-SCAN: A CRISPR/dCas9-based lateral flow assay for rapid, specific, and sensitive detection of SARS-CoV-2. ACS Synth. Biol. 11, 406–419 (2022).
De Puig, H. et al. Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 7, eabh2944 (2021).
Kaminski, M. M. et al. A CRISPR-based assay for the detection of opportunistic infections post-transplantation and for the monitoring of transplant rejection. Nat. Biomed. Eng. 4, 601–609 (2020).
Lee, R. A. et al. Ultrasensitive CRISPR-based diagnostic for field-applicable detection of Plasmodium species in symptomatic and asymptomatic malaria. Proc. Natl Acad. Sci. USA 117, 25722–25731 (2020).
Pardee, K. et al. Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 165, 1255–1266 (2016).
Broto, M. et al. Nanozyme-catalysed CRISPR assay for preamplification-free detection of non-coding RNAs. Nat. Nanotechnol. https://doi.org/10.1038/s41565-022-01179-0 (2022). This article reports the pairing of the ultrasensitive properties of nanozyme detection in lateral flow tests with point-of-care molecular detection enabled by CRISPR.
Fozouni, P. et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 184, 323–333.e9 (2021).
Xiong, E. et al. Simultaneous dual-gene diagnosis of SARS-CoV-2 based on CRISPR/Cas9-mediated lateral flow assay. Angew. Chem. Int. Ed. 60, 5307–5315 (2021).
Soh, J. H., Chan, H.-M. & Ying, J. Y. Strategies for developing sensitive and specific nanoparticle-based lateral flow assays as point-of-care diagnostic device. Nano Today 30, 100831 (2020).
Gray, E. R. et al. Unravelling the molecular basis of high affinity nanobodies against HIV p24: in vitro functional, structural, and in silico insights. ACS Infect. Dis. 3, 479–491 (2017).
Loynachan, C. N. et al. Platinum nanocatalyst amplification: redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 12, 279–288 (2018).
Rodríguez, M. O., Covián, L. B., García, A. C. & Blanco-López, M. C. Silver and gold enhancement methods for lateral flow immunoassays. Talanta 148, 272–278 (2016).
Miller, B. S. et al. Sub-picomolar lateral flow antigen detection with two-wavelength imaging of composite nanoparticles. Biosens. Bioelectron. 207, 114133 (2022).
Wang, Y. et al. Thermal contrast amplification reader yielding 8-fold analytical improvement for disease detection with lateral flow assays. Anal. Chem. 88, 11774–11782 (2016).
Han, G. R., Koo, H. J., Ki, H. & Kim, M. G. Paper/soluble polymer hybrid-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interf. 12, 34564–34575 (2020).
Han, G.-R., Ki, H. & Kim, M.-G. Automated, universal, and mass-producible paper-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interf. https://doi.org/10.1021/acsami.9b17888 (2020).
Li, X. et al. A fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe and lateral flow test strip. Talanta 100, 1–6 (2012).
Wang, J. et al. Quantum dot-based lateral flow test strips for highly sensitive detection of the tetanus antibody. ACS Omega 4, 6789–6795 (2019).
Wei, Q. et al. Fluorescent imaging of single nanoparticles and viruses on a smart phone. ACS Nano 7, 9147–9155 (2013).
Urusov, A. E., Zherdev, A. V. & Dzantiev, B. B. Towards lateral flow quantitative assays: detection approaches. Biosensors 9, E89 (2019).
Tran, V., Walkenfort, B., König, M., Salehi, M. & Schlücker, S. Rapid, quantitative, and ultrasensitive point-of-care testing: a portable SERS reader for lateral flow assays in clinical chemistry. Angew. Chem. Int. Ed. 58, 442–446 (2019).
Hassanain, W. A. et al. Rapid ultra-sensitive diagnosis of Clostridium difficile infection using a SERS-based lateral flow assay. Analyst 146, 4495–4505 (2021).
Hassanain, W. A., Johnson, C. L., Faulds, K., Graham, D. & Keegan, N. Recent advances in antibiotic resistance diagnosis using SERS: focus on the “big 5” challenges. Analyst https://doi.org/10.1039/D2AN00703G (2022).
Kim, N. et al. Surface enhanced Raman scattering artificial nose for high dimensionality fingerprinting. Nat. Commun. 11, 207 (2020).
Lei, H., Wang, K., Ji, X. & Cui, D. Contactless measurement of magnetic nanoparticles on lateral flow strips using tunneling magnetoresistance (TMR) sensors in differential configuration. Sensors 16, E2130 (2016).
Lan, W.-J. et al. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip 13, 4103–4108 (2013).
Huang, S. & Chen, Y. Ultrasensitive fluorescence detection of single protein molecules manipulated electrically on Au nanowire. Nano Lett. 8, 2829–2833 (2008).
Mudanyali, O. et al. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip 12, 2678–2686 (2012).
Ozcan, A. Mobile phones democratize and cultivate next-generation imaging, diagnostics and measurement tools. Lab Chip 14, 3187–3194 (2014).
Hernández-Neuta, I. et al. Smartphone-based clinical diagnostics: towards democratization of evidence-based health care. J. Intern. Med. 285, 19–39 (2019).
Vashist, S. K., Mudanyali, O., Schneider, E. M., Zengerle, R. & Ozcan, A. Cellphone-based devices for bioanalytical sciences. Anal. Bioanal. Chem. 406, 3263–3277 (2014).
Trofymchuk, K. et al. Addressable nanoantennas with cleared hotspots for single-molecule detection on a portable smartphone microscope. Nat. Commun. 12, 950 (2021).
Wei, Q. et al. Plasmonics enhanced smartphone fluorescence microscopy. Sci. Rep. 7, 2124 (2017).
Vashist, S. K., Luppa, P. B., Yeo, L. Y., Ozcan, A. & Luong, J. H. T. Emerging technologies for next-generation point-of-care testing. Trends Biotechnol. 33, 692–705 (2015).
Boeras, D. et al. Evaluation of Zika rapid tests as aids for clinical diagnosis and epidemic preparedness. eClinicalMedicine 49, 101478 (2022).
Roche to launch SARS-CoV-2 & Flu A/B rapid antigen test in countries accepting the CE mark to enable rapid differentiation of viral respiratory infections. Roche https://www.roche.com/media/releases/med-cor-2021-12-06.htm (2021).
Dual HIV/syphilis rapid diagnostic tests can be used as the first test in antenatal care. PAHO/WHO https://www.paho.org/en/documents/dual-hivsyphilis-rapid-diagnostic-tests-can-be-used-first-test-antenatal-care (2019).
Joung, H. A. et al. Point-of-care serodiagnostic test for early-stage lyme disease using a multiplexed paper-based immunoassay and machine learning. ACS Nano 14, 229–240 (2020).
Zhang, T. et al. A paper-based assay for the colorimetric detection of SARS-CoV-2 variants at single-nucleotide resolution. Nat. Biomed. Eng. 6, 957–967 (2022).
Ballard, Z. S. et al. Deep learning-enabled point-of-care sensing using multiplexed paper-based sensors. npj Digit. Med. 3, 66 (2020).
Li, J. & Macdonald, J. Multiplex lateral flow detection and binary encoding enables a molecular colorimetric 7-segment display. Lab Chip 16, 242–245 (2016).
Hofmann, E. R. et al. Blind spot: a Braille patterned novel multiplex lateral flow immunoassay sensor array for the detection of biothreat agents. ACS Omega 6, 22700–22708 (2021).
Wood, C. S. et al. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature 566, 467–474 (2019).
Consortium, A. L. et al. Machine learning for determining lateral flow device results in asymptomatic population: a diagnostic accuracy study. SSRN Electron. J. https://doi.org/10.2139/SSRN.3861638 (2021).
Spiegelhalter, D. J. Probabilistic expert systems in medicine: practical issues in handling uncertainty. Stat. Sci. 2, 25–30 (1987).
Global analysis of health care waste in the context of COVID-19. WHO https://www.who.int/publications/i/item/9789240039612 (2022).
Lia launches ‘world’s first’ flushable pregnancy test. Dezeen https://www.dezeen.com/2017/12/13/lia-launches-worlds-first-flushable-pregnancy-test-design-products/ (2017).
Gray, A. et al. Animal-free alternatives and the antibody iceberg. Nat. Biotechnol. 38, 1234–1239 (2020).
Esquivel, J. P., Del Campo, F. J., Gómez De La Fuente, J. L., Rojas, S. & Sabaté, N. Microfluidic fuel cells on paper: meeting the power needs of next generation lateral flow devices. Energy Environ. Sci. 7, 1744–1749 (2014).
Technical specifications series. WHO — Prequalification of medical products (IVDs, medicines, vaccines and immunization devices, vector control). WHO https://extranet.who.int/pqweb/vitro-diagnostics/technical-specifications-series (2020).
Preferred product characteristics and target product profiles. WHO https://www.who.int/teams/immunization-vaccines-and-biologicals/product-and-delivery-research/ppcs (2022).
Middleton, A. et al. How can we make self-sampling packs for sexually transmitted infections and bloodborne viruses more inclusive? A qualitative study with people with mild learning disabilities and low health literacy. Sex. Transm. Infect. 97, 276–281 (2021).
Guidance for the design of self-sampling packs and associated support for self-sampling processes within sexually transmitted infection and blood borne virus testing. British Association for Sexual Health and HIV https://www.bashhguidelines.org/media/1280/bashhguidanceself-samplingaug2021.pdf (2021).
Devlin, H. The rise of lateral flow tests: are these ‘heroes’ of the pandemic here to stay? The Guardian (8 January 2022).
Straightforward, inexpensive and sensitive. Nat. Biomed. Eng. 6, 923–924 (2022).
Brien, P. & Loft, P. Reducing the UK’s aid spending in 2021. UK Parliament https://commonslibrary.parliament.uk/research-briefings/cbp-9224/ (2022).
OraSure. Gates Foundation https://sif.gatesfoundation.org/investments/orasure/ (2017).
United Nations Sustainable Development Goals: leave no one behind. United Nations https://unsdg.un.org/2030-agenda/universal-values/leave-no-one-behind (2022).
Julie Credou, R. F. & Thomas, B. Photo-assisted inkjet printing of antibodies onto cellulose for the eco2-friendly preparation of immunoassay membranes. RSC Adv. 5, 29786–29798 (2015).
Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. WHO https://www.who.int/publications-detail-redirect/9789240031593 (2021).
Ghebreyesus, T. A. & von der Leyen, U. A global pandemic requires a world effort to end it – none of us will be safe until everyone is safe. WHO https://www.who.int/news-room/commentaries/detail/a-global-pandemic-requires-a-world-effort-to-end-it-none-of-us-will-be-safe-until-everyone-is-safe (30 September 2020).
Access to COVID-19 tools funding commitment tracker. WHO https://www.who.int/publications/m/item/access-to-covid-19-tools-tracker (2022).
Green, M. A. et al. Evaluating social and spatial inequalities of large scale rapid lateral flow SARS-CoV-2 antigen testing in COVID-19 management: an observational study of Liverpool, UK (November 2020 to January 2021). Lancet Reg. Health Eur. 6, 100107 (2021).
Fast track review guidance for COVID-19 studies. NHS Health Research Authority https://www.hra.nhs.uk/covid-19-research/fast-track-review-guidance-covid-19-studies/ (2022).
COVID-19 test directory. FIND https://www.finddx.org/test-directory/ (2022).
Coronavirus (COVID-19) update: FDA authorizes first antigen test to help in the rapid detection of the virus that causes COVID-19 in patients. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-antigen-test-help-rapid-detection-virus-causes (2020).
Coronavirus (COVID-19) update: FDA authorizes antigen test as first over-the-counter fully at-home diagnostic test for COVID-19. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic (2020).
Coronavirus (COVID-19) update: FDA issues new authorization for the BinaxNOW COVID-19 Ag card home test. FDA https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-new-authorization-binaxnow-covid-19-ag-card-home-test (2020).
African Medical Devices Forum. AMDF http://www.amdfnra.org/ (2022).
Acknowledgements
R.A.McK., J.B., B.S.M., D.H., M.M.S., D.P., N.K., C.S.E., M.S., P.S., J.G. and A.M.J. acknowledge support from i-sense: EPSRC IRC in Agile Early Warning Sensing Systems for Infectious Diseases and Antimicrobial Resistance grant EP/R00529X/1; www.i-sense.org.uk. R.A.McK., J.B., and M.M.S. acknowledge the EPSRC (i-sense EPSRC IRC Next Steps Plus: A Smartphone Powered mRNA Sequence Detector grant EP/R018707/1). R.A.McK., M.M.S. and N.K. acknowledge funding from IRC Next Steps Plus: Ultra-Sensitive Enhanced NanoSensing of Anti-Microbial Resistance (uSense) grant EP/R018391/1. A.O. and G.-R.H. acknowledge support from NSF PATHS-UP. M.S. is an NIHR Research Professor (grant NIHR 301634) and also receives funding from the US National Institutes of Health (NIH) (grant 5R01MH114560-03), the Wellcome Trust (grant 082384/Z/07/Z) and the Bill and Melinda Gates Foundation (grant INV-033650). E.J.T. acknowledges support from the NIH (grant NIH UL1TR002550). M.R.T. acknowledges support from grant EP/R00529X/1. R.M., M.F.S. and B.R. acknowledge support from the funders of the Access to COVID-19 Tools Accelerator (ACT-A) (https://www.who.int/publications/m/item/access-to-covid-19-tools-tracker). M.M.S. acknowledges support from the Royal Academy of Engineering Chair in Emerging Technologies award (CiET2021\94). All authors thank A. Byrne for technical proofreading.
Author information
Authors and Affiliations
Contributions
R.A.McK., J.B., B.M., N.E.W., D.C., D.H., N.F., G.-R.H., M.B., C.S.E., J.G., D.P., P.S., R.M., M.R.T., M.F.S., A.O., J.J.C., B.R. and R.W.P. researched data for the article, contributed to the discussion of content, wrote, reviewed and edited the manuscript. A.T.D., N.K., M.M.S., E.N., E.T. and A.M.J. wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.M.S. and M.B. have filed patent application GB2110729.7 relating to nanozyme-catalysed biosensing. M.M.S. has filed patent applications GB2015943.0 and US20200116725 (A1), and is co-inventor on patents ES2365536 (T3) and WO2007063300 (A3) relating to nanomaterials and assays for biosensing. B.S.M. and R.M. have filed patent application WO2020049303A1 (nanodiamond assay). A.O. has pending and issued patents on point-of-care sensors and related technologies. A.M.J. is President of the Academy of Medical Sciences. M.M.S. is a co-founder and director of Signatur Biosciences Ltd. N.E.W. is a cofounder and consults for 52 North Health Ltd. J.J.C. is a co-founder and director of Sherlock Biosciences. E.J.T. is an advisor to Quest Diagnostics. N.F. and R.W.P. are funded by the Wellcome Trust project: The Accelerating Diagnostic Access Project (ADAP). All remaining authors have no competing interests to declare.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Xiaoming Zhou, Nina Papavasiliou, and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Access to COVID-19 tools (ACT): https://www.act-a.org/
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Budd, J., Miller, B.S., Weckman, N.E. et al. Lateral flow test engineering and lessons learned from COVID-19. Nat Rev Bioeng 1, 13–31 (2023). https://doi.org/10.1038/s44222-022-00007-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-022-00007-3
This article is cited by
-
Comparison of pre-labelled primers and nucleotides as DNA labelling method for lateral flow detection of Legionella pneumophila amplicons
Scientific Reports (2024)
-
Falsification of home rapid antigen lateral flow tests during the COVID-19 pandemic
Scientific Reports (2024)
-
From equitable access to equitable innovation: rethinking bioengineering for global health
Nature Reviews Bioengineering (2024)
-
Rapid deep learning-assisted predictive diagnostics for point-of-care testing
Nature Communications (2024)
-
Bioengineering for low-resource settings
Nature Reviews Bioengineering (2023)