Clinical characteristics of 4,520 paediatric patients infected with the SARS-CoV-2 omicron variant, in Xi'an, China
- 1Department of Emergency, Xi’an Children's Hospital (Xi'an Jiaotong University Affiliated Children’s Hospital), Xi'an, Shaanxi, China
- 2Department of Gastroenterology, Xi'an Tus-Children’s Hospital, Shaanxi, Xi'an, China
Background and objective: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has broad tissue tropism and high transmission, which are likely to perpetuate the pandemic. The study aim to analyze the clinicopathogenic characteristics in paediatric patients.
Methods: In this single-centre study, we retrospectively included all confirmed cases infected by SARS-CoV-2 infection at Xi’an Children's Hospital, China, from 1 December to 31 December 2022. The demographic, clinical, laboratory, and radiological features of the patients were analysed.
Results: A total of 4,520 paediatric patients with SARS-CoV-2 omicron variant infections were included. Of these, 3,861 (85.36%) were outpatients, 659 (14.64%) were hospitalised patients, and nine patients (0.20%) died. Of the nine patients who died, five were diagnosed with acute necrotising encephalopathy (ANE). The most common symptoms were fever in 4,275 (94.59%) patients, cough in 1,320 (29.20%) patients, convulsions in 610 (13.50%) patients, vomiting in 410 (9.07%) patients, runny nose/coryza in 277 (6.13%) patients, hoarseness of voice in 273 (6.04%) patients. A blood cell analysis showed a slight elevation of monocytes (mean: 11.14 ± 0.07%). The main diagnoses for both outpatients and inpatients were respiratory infection with multisystem manifestations.
Conclusions: A high incidence of convulsions is a typical characteristic of children infected with SARS-CoV-2. Five of the nine COVID-19 fatalities were associated with ANE. This indicates that nervous system damage in children with SARS-CoV-2 infection is more significant.
1 Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a permanently pandemic of acute respiratory disease (1, 2). “Omicron”, a new variant of the Coronavirus (B.1.1.529) has led to a global pandemic (3). These new BQ and XBB sub-variants of Omicron are transmitted far more rapidly than other variants of SARS-CoV-2 (4–6). SARS-CoV-2 is highly transmissible with broad tissue tropism that is likely perpetuating the pandemic (7). The infection of humans with SARS-CoV-2, lead to severe “flu”-like symptoms that can progress to pneumonia, acute respiratory distress, renal failure, and death (8–10). This study aimed to investigate the clinical characteristics of a large sample of paediatric patients infected with the omicron variant who were diagnosed and treated at our hospital.
2 Materials and methods
2.1 Patient selection
This retrospective, observational, single-centre study was conducted from 1 December to 31 December 2022. A total of 4,520 paediatric patients were infected with the omicron variant at Xi’an Children's Hospital (The Affiliated Children's Hospital of Xi'an Jiaotong University) in Xi'an, China. All patients enrolled in this study were diagnosed according to World Health Organization interim guidance (11). Throatswab specimens from the upper respiratory tract that were obtained from all patients at admission were maintained in viraltransport medium. 2019nCoV was confrmed by realtime RTPCR. The omicron variant was confirmed by whole-genome sequencing performed by the China Disease Control and Prevention Agency. The demographic and clinical characteristics and laboratory test results of the patients were analysed. This study was approved by the Institutional Review Board of the Affiliated Children's Hospital of Xi'an Jiaotong University.
2.2 Clinical data collection
We collected information from the electronic medical records of paediatric patients who tested positive for SARS-CoV-2 nucleic acids. The demographic data included age, weight, and sex. All the patients were from China. The clinical data included diagnosis, clinical signs, symptoms, and treatment. Among them, convulsions, maximum body temperature, and fever duration upon admission were highlighted. The laboratory data included a full blood cell count, hypersensitive C-reactive protein (hs-CRP), liver function, and myocardial enzymes.
2.3 Statistical analysis
Statistical analysis was performed using SPSS software (version 22.0). Continuous variables were described using the mean, median, and range. Additionally, categorical variables were described as frequency rates and percentages.
3 Results
3.1 Demographic and clinical data
A total of 4,520 paediatric patients with SARS-CoV-2 Omicron variant infections were included in this study. Of these, 3,861 (85.36%) were outpatients, 659 (14.64%) were hospitalised patients, and 9 (0.20%) patients died. The proportion of males was 58.14% and females was 41.86%. The age of onset ranged from newborns to 18 years of age; patients aged 1–3 years accounted for the main proportion. The median patient age was 2.08 (0–18) years (Table 1).
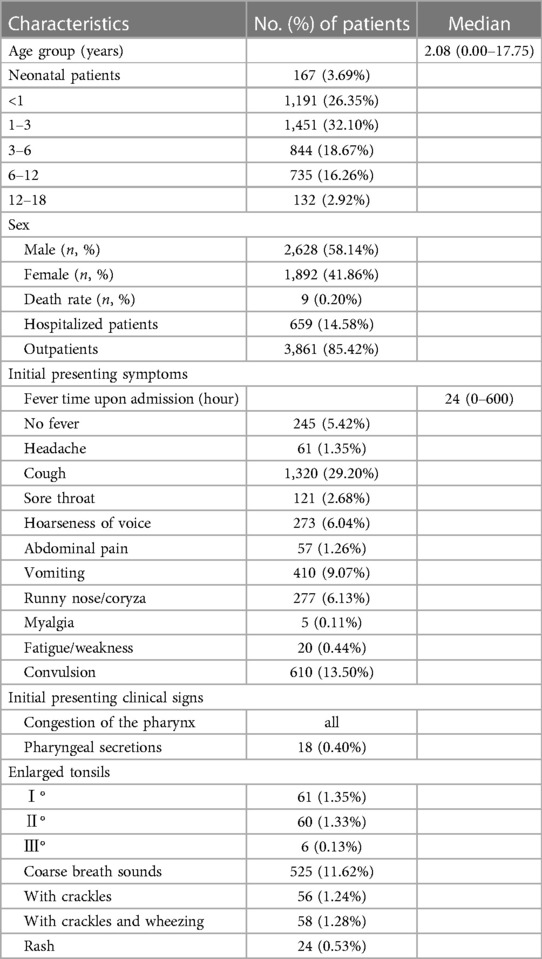
Table 1. Demographics and clinical characteristics of 4,520 patients infected with the omicron variant.
On admission, most patients had fever. Other common symptoms included cough, convulsion, vomiting, runny nose/coryza, hoarseness of voice, sore throat, muscle ache, or headache. The initial presenting clinical signs were mainly involved in respiratory system. The initial presenting symptom was fever in 4,275 patients (94.59%); the median fever time upon admission to the emergency was 24 (0–600) hours. The most common symptoms were cough in 1,320 (29.20%) patients, convulsion in 610 (13.50%) patients, vomiting in 410 (9.07%) patients, runny nose/coryza in 277 (6.13%) patients, hoarseness of voice in 273 (6.04%) patients, sore throat in 121 (2.68%) patients, headache in 61(1.35%) patients, abdominal pain in 57 (1.26%) patients, myalgia in 5 (0.11%) patients, fatigue/weakness in 20 (0.44%) patients, and. The initial presenting clinical signs were congestion of the pharynx in all patients, rash in 24 (0.53%) patients, pharyngeal secretions in 18 (0.40%) patients, enlarged tonsils I° in 61(1.35%) patients, enlarged tonsils Ⅱ° in 60 (1.33%) patients, enlarged tonsils Ⅲ° in 6 (0.13%) patients, coarse breath sounds in 525 (11.62%) patients, patients with crackles 56 (1.24%), and patients with crackles and wheezing 58 (1.28%) (Table 1).
3.2 Laboratory data
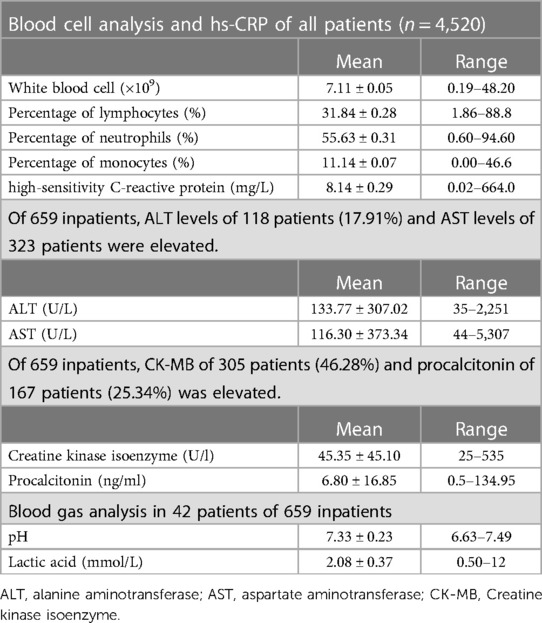
Blood cell analysis was performed in all patients. There was no typical features except the percentage of monocytes was slightly elevated. The mean white blood cell count was 7.11 ± 0.05 (0.19–48.20) × 109; of these, five patients with leukaemia were excluded with a white blood cell count of 118.32–338.72 × 109. The mean percentage of lymphocytes was 31.84 ± 0.28 (1.86–88.8)%; the percentage of neutrophils was 55.63 ± 0.31 (0.60–94.60)%. The percentage of monocytes was 11.14 ± 0.07 (0.00–46.6)%. The mean value of high-sensitivity C-reactive protein was 8.14 ± 0.29 (0.02–664.0) mg/L (Table 2).
Liver function, myocardial enzyme, procalcitonin were often elevated in severe cases,. The normal ranges for alanine aminotransferase (ALT) is 7–35 U/L, aspartate aminotransferase (AST) is 14–44 U/L, creatine kinase isoenzyme is 0–25 U/L, and procalcitonin is 0–0.5 ng/dl. Of 659 inpatients, ALT levels of 118 patients (17.91%) and AST levels of 323 patients were elevated. The mean ALT value was 133.77 ± 307.02 (35–2,251) U/L, AST was 116.30 ± 373.34 (44–5,307) U/L. Of 659 inpatients, creatine kinase isoenzyme (CK-MB) of 305 patients (46.28%) and procalcitonin of 167 patients (25.34%) was elevated. The mean CK-MB was 45.35 ± 45.10 (25–535) U/L; procalcitonin levels were 6.80 ± 16.85 (0.5–134.95) ng/ml. Blood gas analyses were performed in 42 patients. The mean of pH was 7.33 ± 0.23 (6.63–7.49) and lactic acid (LAC) was 2.08 ± 0.37 (0.50–12) mmol/L (Table 2).
3.3 Characteristics of main diagnoses
3.3.1 Characteristics of main diagnoses in outpatients
Upper respiratory tract infection (URTI) was the main diagnosis about the outpatients and accompanied with other manifestations of various system damage, particularly convulsion. Of the 3,861 outpatients, 3,404 patients (88.16%) were diagnosed with upper respiratory tract infection, 379 patients (9.82%) with bronchitis, and 78 patients (2.02%) with pneumonia (Figure 1A). Of the patients with URTI, 1,035 patients presented with concomitant symptoms, such as convulsion (386 cases, 37.29%), laryngitis (224 cases, 21.64%), vomiting (214 cases, 20.68%), abdominal pain (55 cases, 5.31%), diarrhoea (46 cases, 4.44%), acute gastroenteritis (11 cases, 1.06%), rash (21 cases, 2.03%), enlarged tonsils (39 cases, 3.77%), tonsils with secretions (20 cases, 1.93%), thrombocytopenic purpura (6 cases, 0.58%), allergic reaction (2 cases, 0.19%), sepsis (2 cases, 0.19%), haematuria (1 case, 0.10%), and underlying diseases (8 cases, 0.77%) (Figure 1B). Among the bronchitis patients, 32 patients presented with concomitant symptoms such as convulsions (6 cases, 18.75%), hoarseness of voice (19 cases, 59.38%), vomiting (3 cases, 9.38%), tonsils with secretions (1 case, 3.13%), thrombocytopenic purpura (1 case, 3.13%), myocardial damage (1 case, 3.13%) (Figure 1C), and underlying diseases (1 case, 3.13%). Of the patients with pneumonia, 3 patients presented with concomitant symptoms, such as diarrhoea (1 case, 33.33%), pulmonary emphysema (1 case, 33.33%), and underlying diseases (1 case, 33.33%) (Figure 1D).
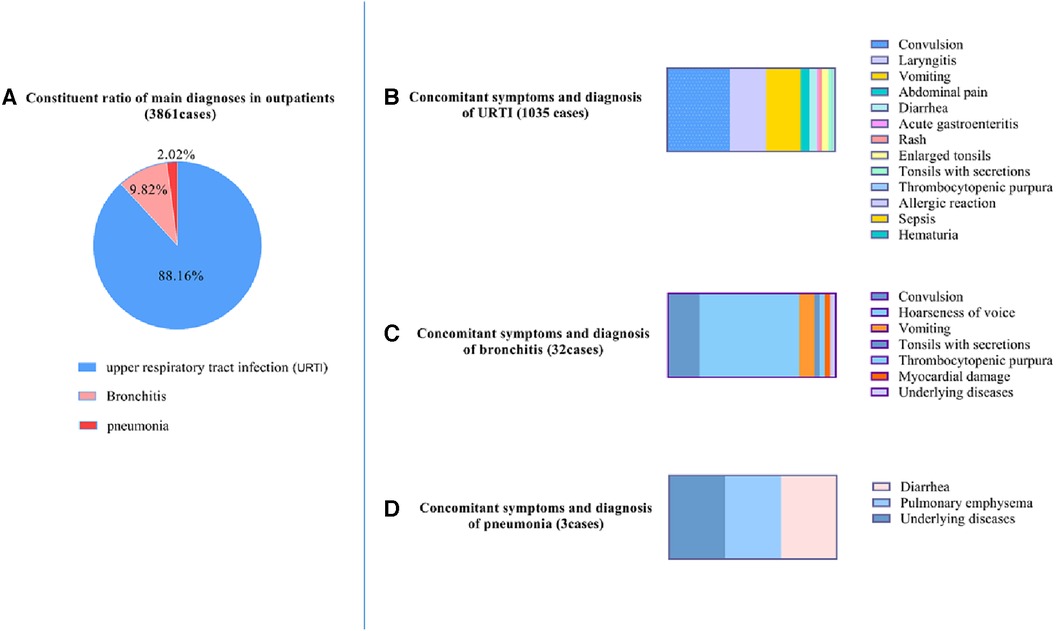
Figure 1. Characteristics of main diagnoses and concomitant symptoms in outpatients. (A) Constituent ratio of main diagnoses in outpatients; (B) upper respiratory tract infection (URTI) with concomitant symptoms. (C) Bronchitis with concomitant symptoms. (D) Pneumonia with concomitant symptoms.
3.3.2 Characteristics of main diagnoses in inpatients
Pneumonia, URTI and bronchitis were the main diagnosis about the inpatients accompanied with multiple organ damage. Of the 659 inpatients, 171 patients (25.95%) were diagnosed with URTI; 101 patients (15.33%) had bronchitis; 239 patients (36.27%) had pneumonia (Figure 2); 18 had viral encephalitis; 7 had toxic encephalopathy; 5 had acute necrotising encephalopathy (ANE); 1 had cerebral infarction; 1 had cerebrospinal meningitis; 18 had seizures; 13 had sepsis; 1 had fulminant myocarditis; 1 had arrhythmology; 5 were diagnosed with Kawasaki disease; 9 had vomiting and diarrhoea; 2 had gastrointestinal bleeding; 2 had allergic purpura; 10 patients had immune thrombocytopenia; 10 patients were nephrotic and had nephritis; 2 had systemic lupus erythematosus (SLE) nephritis; 2 patients had diabetes; 7 had leukaemia and aplastic anaemia; 11 had appendicitis; 7 had surgical disease; and 9 patients had complicated underlying diseases. Febrile seizures occurred mainly in patients with URTI (84 cases, 49.12%), bronchitis (35 cases, 34.65%), and pneumonia (11 cases, 4.60%) (Figure 2). Myocardial damage also occurred mainly in URTI (10 cases, 5.85%), bronchitis (12 cases, 11.88%), and pneumonia (11 cases, 7.53%) (Figure 2).
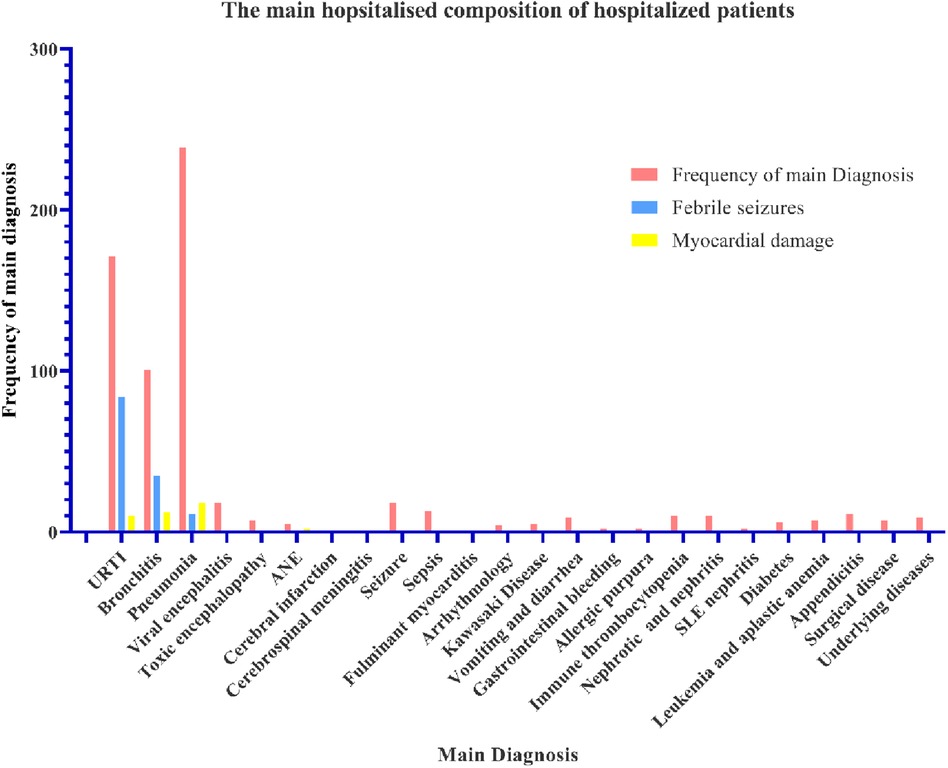
Figure 2. The main diagnostic composition of hospitalised patients. The abscissa is the diagnosis and accompanying symptoms. The ordinate is the frequency of the patients.
3.3.3 Characteristics of main diagnoses in patients who died
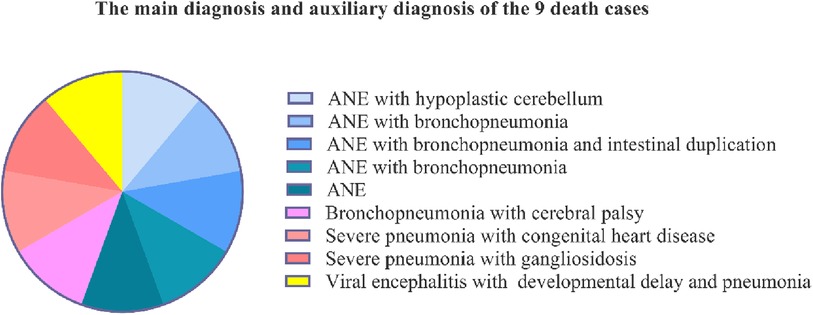
Of the nine patients who died, five were diagnosed with acute necrotising encephalopathy (ANE); two cases were associated with congenital malformations, which were hypoplastic cerebellum and intestinal duplication, and three cases were associated with bronchopneumonia. Of these patients, three were diagnosed with bronchopneumonia or severe pneumonia associated with cerebral palsy, congenital heart disease, and gangliosidosis, and one had viral encephalitis with developmental delay and pneumonia (Figure 3).
3.4 Clinical features of the patients who died (n = 9)
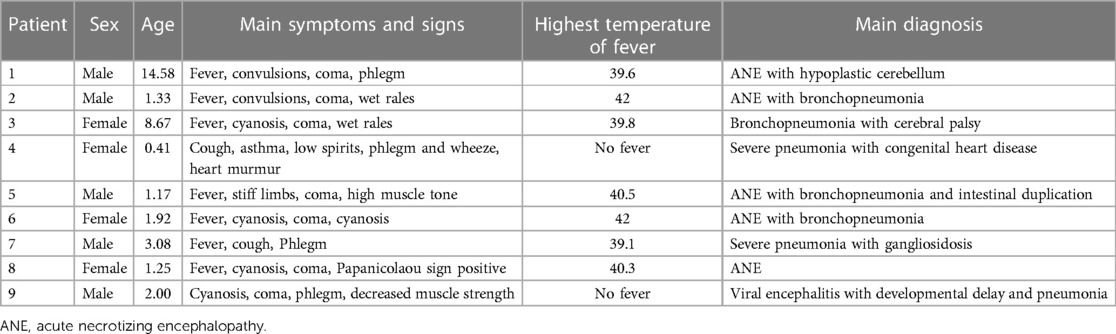
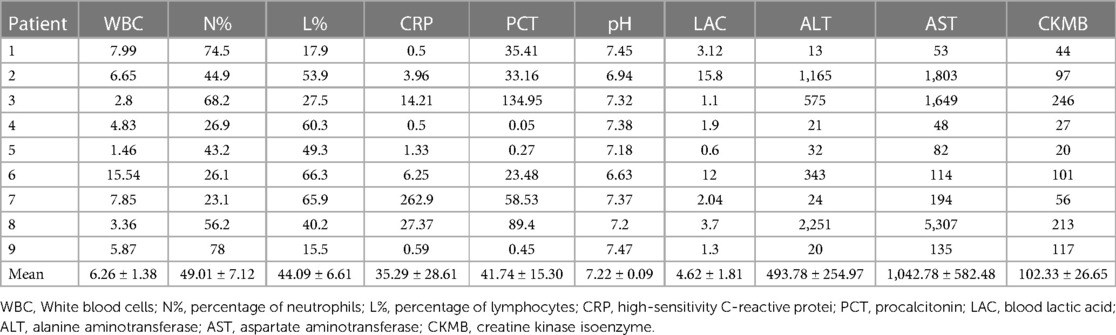
The main early symptoms and signs of ANE were high or very high fever, convulsions, and coma. The procalcitonin (PCT) level was obviously elevated, which is uncommon in viral infections. Of the nine patients who died, five were male and four were female. The median patient age was 1.92 (0.41–14.58) years. The main symptoms of pneumonia, were cough, cyanosis, phlegm, wet rales, and wheezing (Table 3). The blood cell analysis results were unremarkable. High-sensitivity C-reactive protein (CRP) levels were elevated in only one case of severe pneumonia. However, the procalcitonin (PCT) level was obviously elevated in six patients (mean: 41.74 ± 15.30). The pH was also markedly abnormal in six patients (mean: 7.22 ± 0.09). Blood lactic acid (LAC) was obviously elevated in five patients (mean: 4.62 ± 1.81), alanine aminotransferase (ALT) in four patients (mean: 493.78 ± 254.97), aspartate aminotransferase (AST) in seven patients (mean: 1,042.78 ± 582.48), and creatine kinase isoenzyme (CK-MB) in five patients (mean: 102.33 ± 26.65) (Table 4).
In brain nuclear magnetic resonance imaging (MRI), bilateral thalamic symmetrical damage were observed in all patients with ANE. The typical lesion showed restricted diffusion in the thalami and swelling and increased T2-weighted signal (Figures 4A,B: red arrows). Symmetrical, multifocal thalamic damage is distinctive of ANE. It always involves the brainstem and cerebellum (Figure 4A: black arrow), and the parietal cortex (Figure 4A: yellow arrow), periventricular white matter, and the corpus callosum (Figure 4B: yellow arrow), and basal ganglia (Figure 4B: white arrow), and external and internal capsule injuries (Figure 4A: white arrow), and the centrum semiovale (Figure 4B: black arrow), temporal lobe, and amygdala. All patients had bilaterally symmetrical lesions, often showing as cytotoxic oedema, ischaemia, and necrotising changes in the lesion area.
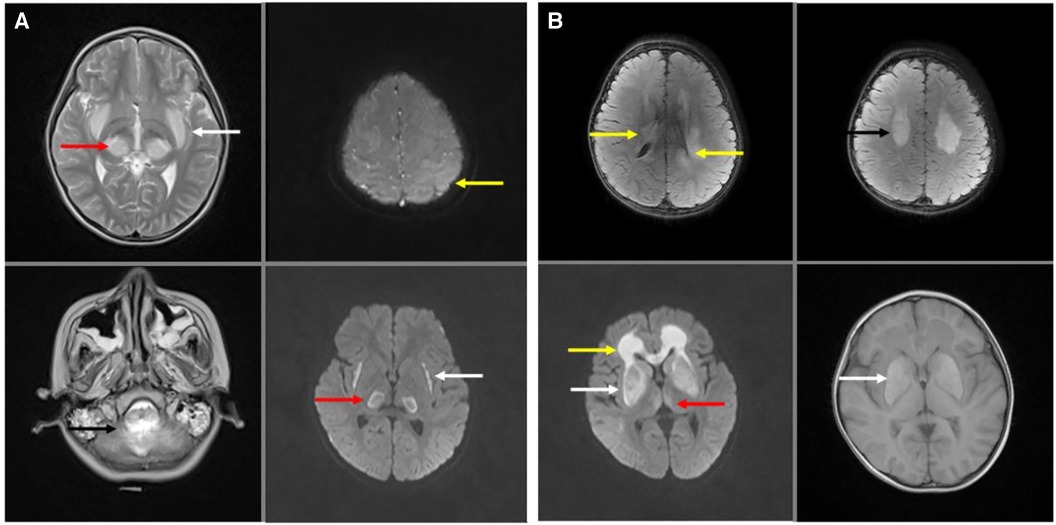
Figure 4. Brain nuclear magnetic resonance (MRI) of acute necrotizing encephalopathy. (A) Bilateral thalamic symmetrical damage in T2- weighted and T2-weighted flair (red arrow). Brain stem and cerebellum injured in T2- weighted (black arrow). The cerebral cortex was extensively oedematous with significant damage to the left parietal cortex (yellow arrow) in T2-weighted flair. External and internal capsule injury in T2-weighted flair (white arrow). (B) Bilateral thalamic symmetrical damage in T2-weighted flair (red arrow). Periventricular white matter damage in T2-weighted flair (yellow arrow). Centrum semiovale damaged in T2-weighted flair (black arrow). Basal ganglia damaged in T2-weighted flair and T1-weighted (white arrow).
3.4 Spearman's correlation analyses between lab findings with clinical diagnoses
Spearman's correlation coefficient was calculated to determine a statistically significant correlation between the lab findings with clinical diagnoses. AST and CK-MB were significant negatively correlated with ANE (AST: rs = −0.140, CK-MB: rs = −0.148, p < 0.001) and death (AST: rs = −0.160, CK-MB: rs = −0.148, p < 0.001). AST were significant positively correlated with frequency of convulsions (rs = 0.155, p < 0.001). ALT were significant negatively correlated with ANE (rs = −0.134, p = 0.001) and negatively correlated with death (rs = −0.160, p = 0.01). PCT were significant negatively correlated with death (rs = −0.139, p = 0.001) and negatively correlated with ANE (rs = −0.081, p = 0.045). Percentage of monocytes were significant positively correlated with death (rs =0.129, p = 0.001). The correlation result show that children with SARS-CoV-2 Omicron Variant infection had higher levels of AST, ALT, CK-MB and PCT correlated with more risk of ANE and death. Elevated AST maybe was a predictors of convulsions. Elevated percentage of monocytes maybe was a protective factors of death (Figure 5).
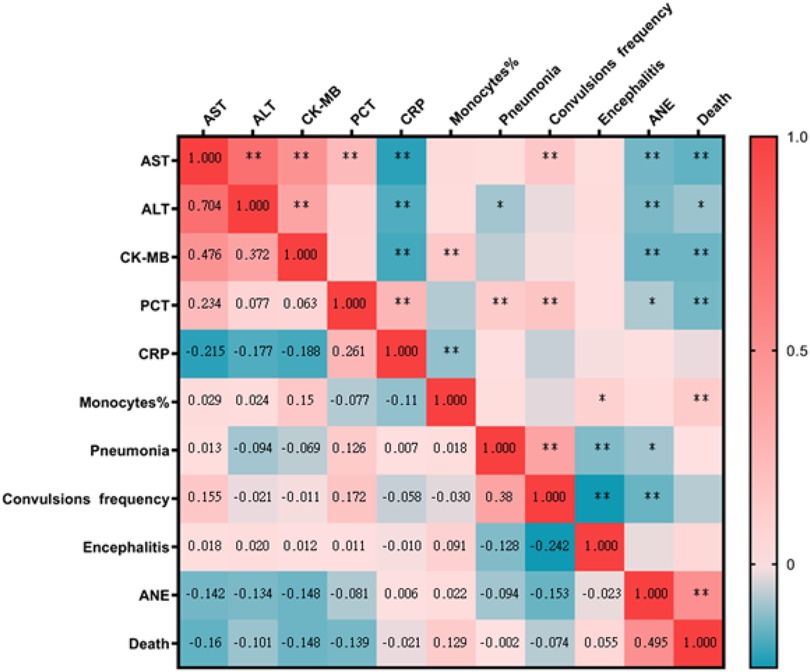
Figure 5. A heat map of Spearman's correlation analyses between lab findings with clinical diagnoses. *, correlation; **, Significant correlation. AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK-MB, creatine kinase isoenzyme; PCT, procalcitonin; CRP, hypersensitive C-reactive protein; Monocytes%, percentage of monocytes; ANE, acute necrotizing encephalopathy.
3.5 Predictive factors for severe disease requiring hospitalization
A logistic analysis was performed. The results showed that fever time upon admission (hour), peak fever, WBC, neutrophils percentage, CRP and convulsion frequency were independent risk factors for hospitalization of children with Omicron Variant infection (P < 0.05). The odds ratio values were 1.022, 0.943, 1.072, 0.976, 1.005 and 2.844 respectively as shown in Table 5.
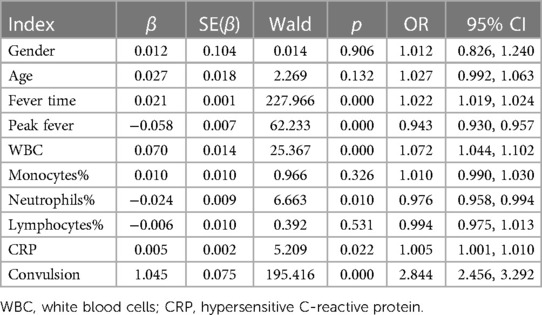
Table 5. Logistic analysis for the related factors predicting hospitalization of children with omicron variant infection.
4 Discussion
Strong natural immunity is acquired after the primary infection by SARS-CoV-2 and may last for more than one year (12). However, Nguyen et al. reported that the severity of the second SARS-CoV-2 infection was similar to that of the first infection (13). Therefore, it is important to understand the pathogenic characteristics of SARS-CoV-2 in children. This was a descriptive study on the clinical characteristics of COVID-19, including data on 4,520 paediatric patients with Omicron variant infections. The mortality rate was 0.20% and the rate of hospitalization was 14.64%.
The aetiopathogenesis of SARS-CoV-2 infection in humans is reveals itself as mild symptoms to severe respiratory failure (14). In this study, 3,861 (85.36%) patients were outpatients with mild symptoms. The initial and main presenting symptom was a fever. The most common accompanying symptoms were sequent to cough (29.20%), convulsions (13.50%), vomiting (9.07%), hoarseness of voice (6.04%). The most typical symptom is a significant increase in convulsions. This indicates that nervous system damage in children with SARS-CoV-2 infection is more significant.
Of the nine patients who died, five were diagnosed with acute necrotising encephalopathy (ANE), of which the main symptoms and signs were high fever or extremely high fever, convulsions, and coma; two cases were associated with hypoplastic cerebellum and intestinal duplication; three cases were associated with bronchopneumonia; one was viral encephalitis with developmental delay and pneumonia. Six (66.67%) patients died of damage to the central nervous system. The pathogenesis is unknown. In a recent animal study, SARS-CoV-2 infections caused encephalitis in mouse models, which is not common in adult patients with SARS-CoV-2 (15–17), but was found in paediatric patients in our study. During early infection, viral replication occurs in multiple respiratory and non-respiratory tissues, including the brain (18). Varga et al. found evidence of direct SARS-CoV-2 infection of the endothelial cell by the ACE2 (angiotensin converting enzyme 2) receptor and diffuse endothelial inflammation (19, 20). It can also act as a receptor for SARS-COV-2, mediating viral entry into cerebral cells and spreading to the infective area (20). The SARS-COV-2 uses the ACE2 receptor expressed by alveolar epithelial cells to infect the host, causes lung injury, and recruits immune cells (19), which triggers a strong immune response known as cytokine storm syndrome (14). The “cytokine storm” can lead to widespread endothelial dysfunction and apoptosis in multiple organs (19). It is hypothesised that this mechanism also exists in cerebral vessels. When the cerebral vascular endothelial cells are injured, thrombus formation and occlusion can easily occur. In this study, all ANE cases showed ischaemia and necrotising changes in the basal ganglia, which is consistent with this mechanism. They often present as bilateral symmetrical lesions typically in the thalamus. The uniform and symmetrical distribution damage due to the energy depletion state (21, 22). This is a dynamic process that corresponds to clinical and pathophysiological changes, such as: cerebral edema-pitting, hemorrhage, necrosis-degenerative changes (23). Some patients may experience complete regression of the lesions. But others may have residual lesions such as atrophy, white matter cyst, hypothalamic density, and hemosiderin deposition (21, 24). Further studies are required to investigate the mechanisms of symmetrical damage.
Among the 659 inpatients, the main diagnoses were pneumonia (36.27%), URTI (25.95%), and bronchitis (15.33%). Common diagnoses were viral encephalitis, seizure, sepsis, appendicitis, immune thrombocytopenia, nephrosis and nephritis, vomiting and diarrhoea, complicated underlying diseases, encephalopathy, leukaemia and aplastic anaemia, surgical disease, ANE, Kawasaki disease. Rare diagnoses included gastrointestinal bleeding, allergic purpura, systemic lupus erythematosus, nephritis, diabetes, cerebral infarction, cerebrospinal meningitis, fulminant myocarditis, and arrhythmia. This indicates that decease easily lead to multiple organ dysfunctions (25, 26). Febrile seizures occurred mainly in URTI (84 cases, 49.12%), bronchitis (35 cases, 34.65%), and pneumonia (11 cases, 4.60%). Myocardial damage often occurred. The clinical characteristics of the disease include respiratory infections that can invade various organs and systems. A high incidence of febrile seizures is a typical characteristic of children infected with SARS-CoV-2. However, further studies are required to elucidate the underlying pathogenic mechanisms.
Of the 3,861 outpatients, the main diagnoses were URTI (88.16%), bronchitis (9.82%), and pneumonia (2.02%). Among the 1,035 patients with URTI, the patients presented with convulsion (37.29%). Among the 32 patients with bronchitis, the patients presented with convulsions (18.75%). The clinical characteristics of outpatients were respiratory infections with various systemic injuries and a high incidence rate of febrile seizures.
Upon comparison of adult patients with children with SARS-CoV-2 infections, the injuries in adults were mainly in the respiratory and cardiovascular systems, whereas the injuries in children were in the respiratory and nervous systems. The pathogenic characteristics in children are particularly similar to mouse model research (17). Although adult patients with SARS-CoV-2 infections presents a wide variety of neurological manifestations that range from mild to severe symptoms, such as anosmia, dysgeusia, myalgia, headache, hallucinations, psychomotor agitation, encephalopathy, vertigo, brain haemorrhage, brain ischaemia and encephalitis (27–29), the most common causes of death are ARDS, severe viral pneumonia, and multiple organ failure (30, 31). However, differences in pathogenicity between adults and children remain unknown. The mortality rates of hospitalised patients (in our study 9/659: 1.37%) in children are much lower than in adults (4.3%) (8), and in general, the younger the age, the milder the disease presentation.
The blood cell analysis had no typical features except the percentage of monocytes was slightly elevated. Among 659 inpatients patients, ALT was elevated in 17.91% of patients; AST was elevated in 49.01% of patients, and CK-MB were elevated in 46.28% of patients. This indicates that SARS-CoV-2 infection can cause multiple organ dysfunction (25, 26). PCT levels were elevated in 25.34% patients; however, this phenomenon is rare in viral infections, and the mechanism is not known. The limitations of this study are the short period of cases and the single-center study. The long-term 2019-ncov infection effects of children are still unclear, and further in-depth studies are urgently needed.
The Spearman's correlation analyses result show that children with SARS-CoV-2 Omicron Variant infection had higher levels of AST, ALT, CK-MB and PCT correlated with more risk of ANE and death. ALT, AST and CK-MB are important clinical indicators of disease severity. Regulated by endotoxins and cytokines, PCT may also be triggered by widespread tissue damage and endothelial dysfunction (32). The logistic analyses result showed that fever time upon admission (hour), peak fever, WBC, neutrophils percentage, CRP and convulsion frequency were independent risk factors for hospitalization of children with Omicron Variant infection. There is a relative paucity of pediatric data in the management of COVID-19 (33). Our research data will be useful for future research.
Summary, in the study, our findings is meaningful for further research of COVID-19 pathogenesis in children. The complex comorbidities, particularly nervous system damage are the clinical characteristic of pediatric patients with SARS-CoV-2 Omicron Variant infection. It is benefit to clinical management the severe coronavirus disease 2019 in in pediatric populations. Compare to previous pediatric COVID-19 literature, the same finding was neurologic diseases were significantly higher, with the high occurrence of seizures (34). But we analyzed the characteristics of the fatalities and showed imaging features of acute necrotizing encephalopathy. This study has several limitations. First, since this study was conducted in a limited number of big Children's hospital, there was potential selection bias. Second, due to the retrospective review of medical records, the judgment of the severity of illness was potentially misclassified and miss useful information. Finally, our data do not represent the national situation. Might prospective multicenter studies can analysis risk factors of the acute necrotizing encephalopathy, which lead main causes of death in pediatric patients with SARS-CoV-2 Omicron Variant infection. It is aim to provide a basis for early identification and treatment.
5 Conclusions
COVID-19 initially presents with “flu”-like symptoms. The most common symptom in children is a significant increase in the number of convulsions. If there is a high or ultra-high fever and progressive disturbance of consciousness, it is necessary to be alert to the occurrence of encephalitis, which can progress to life-threatening systemic inflammation and multi-organ dysfunction. Acute necrotising encephalopathy and pneumonia with comorbidities are the main causes of death in children with SARS-CoV-2 infection. High incidence of febrile seizures is a typical clinical characteristic. This indicates that nervous system damage in children with SARS-CoV-2 infection is more significant. It is significantly different from that in adults who die of respiratory distress syndrome (white lungs).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Affiliated Children's Hospital of Xi'an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JY: Conceptualization, Investigation, Software, Writing – original draft. JC: Data curation, Formal Analysis, Project administration, Supervision, Validation, Writing – original draft. LL: Data curation, Investigation, Resources, Validation, Writing – original draft. LY: Data curation, Investigation, Resources, Validation, Writing – original draft. ML: Conceptualization, Funding acquisition, Methodology, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Natural Science Foundation of Shaanxi Province, China (No. 2022JM-606) and the hospital program at the Affiliated Children's Hospital of Xi'an Jiaotong University, China (No. 2021D04).
Acknowledgments
We thank all patients involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci. (2021) 25(24):8012–8. doi: 10.26355/eurrev_202112_27652
2. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19(3):141–54. doi: 10.1038/s41579-020-00459-7
3. World Health Organization. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated 2021. (accessed December 22, 2021)).
4. Ingraham NE, Ingbar DH. The omicron variant of SARS-CoV-2: understanding the known and living with unknowns. Clin Transl Med. (2021) 11(12):e685. doi: 10.1002/ctm2.685
5. Wang Q, Iketani S, Li Z, Liu L, Guo Y, Huang Y, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. (2023) 186(2):279–286.e278. doi: 10.1016/j.cell.2022.12.018
6. UK Health Security Agency. SARS-CoV-2 variants of concern and variants under investigation in England. Updated 2021. (accessed December 22, 2021).
7. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. (2020) 41(12):1100–15. doi: 10.1016/j.it.2020.10.004
8. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323(11):1061–9. doi: 10.1001/jama.2020.1585
9. Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. (2020) 395(10223):514–23. doi: 10.1016/S0140-6736(20)30154-9
10. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395(10223):507–13. doi: 10.1016/S0140-6736(20)30211-7
11. Organization WH. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. In. Vol COVID-19: Clinical care.Published January 12, 2020: Availeble online at: https://www.who.int/publications/i/item/10665-332299 (accessed July 01, 2023).
12. Flacco ME, Acuti Martellucci C, Baccolini V, De Vito C, Renzi E, Villari P, et al. Risk of reinfection and disease after SARS-CoV-2 primary infection: meta-analysis. Eur J Clin Invest. (2022) 52(10):e13845. doi: 10.1111/eci.13845
13. Nguyen NN, Houhamdi L, Hoang VT, Delerce J, Delorme L, Colson P, et al. SARS-CoV-2 reinfection and COVID-19 severity. Emerg Microbes Infect. (2022) 11(1):894–901. doi: 10.1080/22221751.2022.2052358
14. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
15. Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, et al. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol. (2007) 81(3):1162–73. doi: 10.1128/JVI.01702-06
16. Winkler ES, Bailey AL, Kafai NM, Nair S, McCune BT, Yu J, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. (2020) 21(11):1327–35. doi: 10.1038/s41590-020-0778-2
17. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. (2008) 82(15):7264–75. doi: 10.1128/JVI.00737-08
18. Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. (2022)612(7942):758–63. doi: 10.1038/s41586-022-05542-y
19. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
20. Wang J, Zhao H, An Y. ACE2 Shedding and the role in COVID-19. Front Cell Infect Microbiol. (2021) 11:789180. doi: 10.3389/fcimb.2021.789180
21. Qin N, Wang J, Peng X, Wang L. Pathogenesis and management of acute necrotizing encephalopathy. Expert Rev Neurother. (2023) 23(7):641–50. doi: 10.1080/14737175.2023.2224503
22. Bloch C, Suter B, Fischmann A, Gensicke H, Rüegg S, Weisser M. Only a touch of the flu? The simultaneous manifestation of acute necrotizing encephalopathy in two consanguineous patients. Open Forum Infect Dis. (2015) 2(2):ofv013. doi: 10.1093/ofid/ofv013
23. Wu L, Peng H, Jiang Y, He L, Jiang L, Hu Y. Clinical features and imaging manifestations of acute necrotizing encephalopathy in children. Int J Dev Neurosci. (2022) 82(5):447–57. doi: 10.1002/jdn.10201
24. Levine JM, Ahsan N, Ho E, Santoro JD. Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult Scler Relat Disord. (2020) 43:102194. doi: 10.1016/j.msard.2020.102194
25. Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. (2020) 251(3):228–48. doi: 10.1002/path.5471
26. Salamanna F, Maglio M, Landini MP, Fini M. Body localization of ACE-2: on the trail of the keyhole of SARS-CoV-2. Front Med (Lausanne). (2020) 7:594495. doi: 10.3389/fmed.2020.594495
27. Chou SH, Beghi E, Helbok R, Moro E, Sampson J, Altamirano V, et al. Global incidence of neurological manifestations among patients hospitalized with COVID-19-A report for the GCS-NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. (2021) 4(5):e2112131. doi: 10.1001/jamanetworkopen.2021.12131
28. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. (2020) 194:105921. doi: 10.1016/j.clineuro.2020.105921
29. Zapa-Pérez N, Moreno-Vargas E, Sierra-Villalobos V, Castro-Salcedo C, Mesa-Santamaria K, Jímenez-Monsalve CA. Neurological symptoms associated with SARS-CoV-2 infection in a tertiary hospital in bogota. Colombia. Neurol Perspect. (2022) 2(2):67–72. doi: 10.1016/j.neurop.2021.12.003
30. Yang B, Xu LY, Li LY, Qiao DF, Du SH, Yue X, et al. Pathological changes and cause of death associated with the global novel coronavirus disease (COVID-19). Fa Yi Xue Za Zhi. (2023) 39(6):586–95. doi: 10.12116/j.issn.1004-5619.2023.430703
31. Xie NN, Zhang WC, Chen J, Tian FB, Song JX. Clinical characteristics, diagnosis, and therapeutics of COVID-19: a review. Curr Med Sci. (2023) 43(6):1066–74. doi: 10.1007/s11596-023-2797-3
32. Matwiyoff GN, Prahl JD, Miller RJ, Carmichael JJ, Amundson DE, Seda G, et al. Immune regulation of procalcitonin: a biomarker and mediator of infection. Inflamm Res. (2012) 61(5):401–9. doi: 10.1007/s00011-012-0439-5
33. Zhu F, Ang JY. COVID-19 Infection in children: diagnosis and management. Curr Infect Dis Rep. (2022) 24(4):51–62. doi: 10.1007/s11908-022-00779-0
Keywords: omicron, coronavirus, SARS-CoV-2, COVID-19, acute necrotizing encephalopathy
Citation: Yue J, Cao J, Liu L, Yin L and Li M (2024) Clinical characteristics of 4,520 paediatric patients infected with the SARS-CoV-2 omicron variant, in Xi'an, China. Front. Pediatr. 12:1325562. doi: 10.3389/fped.2024.1325562
Received: 21 October 2023; Accepted: 30 January 2024;
Published: 23 February 2024.
Edited by:
San-Nan Yang, E-Da Hospital, TaiwanReviewed by:
Yung-Ning Yang, Eda Hospital, TaiwanPei Ling Wu, Eda Hospital, Taiwan
Marwa Arbi, Pasteur Institute of Tunis, Tunisia
© 2024 Yue, Cao, Liu, Yin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingyue Li yaoaimingyue@163.com
 Jingwei Yue1
Jingwei Yue1  Mingyue Li
Mingyue Li