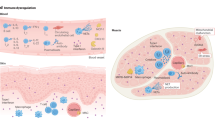
Abstract
Myositis-specific autoantibodies (MSAs) have become pivotal biomarkers for idiopathic inflammatory myopathies and have revolutionized understanding of the heterogeneous disease spectrum that affects both adults and children. The discovery and characterization of MSAs have substantially enhanced patient stratification based on clinical phenotype, thereby facilitating more precise diagnosis and ultimately improving management strategies. Advances in immunoassay technologies in the past 20 years have further propelled the field forward, enabling the detection of a growing repertoire of autoantibodies with high specificity and sensitivity; however, evolving research over the past decade has revealed that even within antibody-defined subsets, considerable clinical diversity exists, suggesting a broader spectrum of disease manifestations than previously acknowledged. Challenges persist, particularly among patients who are seronegative, where the failure to identify certain rare MSAs stems from the use of diverse detection methodologies and inadequate consensus-guided standardization and validation protocols. Bridging these diagnostic gaps is crucial for optimizing patient care and refining prognostic stratification in idiopathic inflammatory myopathies.
Key points
-
Autoantibodies, found in over half of adult and paediatric patients with idiopathic inflammatory myopathies (IIMs), correlate with specific clinical phenotypes, aiding in the classification, diagnosis and prognostic assessment of these diseases.
-
The emergence of new myositis autoantibodies, along with a deeper understanding of serological categories in the past 10 years, enriches the diagnostic and prognostic repertoire for IIMs.
-
A more concerted global exploration of ethnic, environmental and genetic diversities is crucial to improve the characterization of subsets within the serological categories already known in IIMs.
-
Numerous new immunoassays for detecting myositis-specific autoantibodies, including line blot techniques, have variable performance and lack standardized protocols, requiring further validation for reliable myositis autoantibody detection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292, 344–347 (1975).
Bohan, A. & Peter, J. B. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292, 403–407 (1975).
Dalakas, M. C. Polymyositis, dermatomyositis, and inclusion-body myositis. N. Engl. J. Med. 325, 1487–1498 (1991).
Hoogendijk, J. E. et al. 119th ENMC international workshop: trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10–12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 14, 337–345 (2004).
Love, L. A. et al. A new approach to the classification of idiopathic inflammatory myopathy: myositis-specific autoantibodies define useful homogeneous patient groups. Medicine 70, 360–374 (1991).
Betteridge, Z. et al. Frequency, mutual exclusivity and clinical associations of myositis autoantibodies in a combined European cohort of idiopathic inflammatory myopathy patients. J. Autoimmun. 101, 48–55 (2019).
Tansley, S. L. et al. Autoantibodies in juvenile-onset myositis: their diagnostic value and associated clinical phenotype in a large UK cohort. J. Autoimmun. 84, 55–64 (2017).
McHugh, N. J. & Tansley, S. L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 14, 290–302 (2018).
Betteridge, Z. & McHugh, N. Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J. Intern. Med. 280, 8–23 (2016).
Papadopoulou, C., Chew, C., Wilkinson, M. G. L., McCann, L. & Wedderburn, L. R. Juvenile idiopathic inflammatory myositis: an update on pathophysiology and clinical care. Nat. Rev. Rheumatol. 19, 343–362 (2023).
Muro, Y., Ishikawa, A., Sugiura, K. & Akiyama, M. Clinical features of anti-TIF1-α antibody-positive dermatomyositis patients are closely associated with coexistent dermatomyositis-specific autoantibodies and anti-TIF1-γ or anti-mi-2 autoantibodies. Rheumatology 51, 1508–1513 (2012).
Huang, H. L. et al. Coexistence of multiple myositis-specific antibodies in patients with idiopathic inflammatory myopathies. J. Clin. Med. 11, 6972 (2022).
Gupta, L., Naveen, R., Gaur, P., Agarwal, V. & Aggarwal, R. Myositis-specific and myositis-associated autoantibodies in a large Indian cohort of inflammatory myositis. Semin. Arthritis Rheum. 51, 113–120 (2021).
Lundberg, I. E., Miller, F. W., Tjärnlund, A. & Bottai, M. Diagnosis and classification of idiopathic inflammatory myopathies. J. Intern. Med. 280, 39–51 (2016).
Reichlin, M. & Mattioli, M. Description of a serological reaction characteristic of polymyositis. Clin. Immunol. Immunopathol. 5, 12–20 (1976).
Sato, S. et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum. 60, 2193–2200 (2009).
Sato, S. et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum. 52, 1571–1576 (2005).
Fujimoto, M. et al. Myositis-specific anti-155/140 autoantibodies target transcription intermediary factor 1 family proteins. Arthritis Rheum. 64, 513–522 (2012).
Targoff, I. N. et al. A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 54, 3682–3689 (2006).
Gunawardena, H. et al. Autoantibodies to a 140-kd protein in juvenile dermatomyositis are associated with calcinosis. Arthritis Rheum. 60, 1807–1814 (2009).
Betteridge, Z., Gunawardena, H., North, J., Slinn, J. & McHugh, N. Identification of a novel autoantibody directed against small ubiquitin-like modifier activating enzyme in dermatomyositis. Arthritis Rheum. 56, 3132–3137 (2007).
Van Gompel, E. et al. Autoantibodies against the melanoma differentiation-associated protein 5 in patients with dermatomyositis target the helicase domains. Rheumatology 63, 1466–1473 (2024).
Pinal-Fernandez, I. et al. Pathological autoantibody internalisation in myositis. Ann. Rheum. Dis. 83, 1549–1560 (2024).
Nombel, A., Fabien, N. & Coutant, F. Dermatomyositis with anti-MDA5 antibodies: bioclinical features, pathogenesis and emerging therapies. Front. Immunol. 12, 773352 (2021).
Mamyrova, G. et al. Anti-MDA5 autoantibodies associated with juvenile dermatomyositis constitute a distinct phenotype in North America. Rheumatology 60, 1839–1849 (2021).
Ueki, M. et al. Myositis-specific autoantibodies in Japanese patients with juvenile idiopathic inflammatory myopathies. Mod. Rheumatol. 29, 351–356 (2019).
Narang, N. S., Casciola-Rosen, L., Li, S., Chung, L. & Fiorentino, D. F. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res. 67, 667–672 (2015).
Lu, X., Peng, Q. & Wang, G. Anti-MDA5 antibody-positive dermatomyositis: pathogenesis and clinical progress. Nat. Rev. Rheumatol. 20, 48–62 (2024).
Yamasaki, Y. et al. Clinical impact of myositis-specific autoantibodies on long-term prognosis of juvenile idiopathic inflammatory myopathies: multicentre study. Rheumatology 60, 4821–4831 (2021).
Liu, L. et al. Predictors of mortality for dermatomyositis patients positive with anti-melanoma differentiation-related gene 5 and optimal treatment. Clin. Exp. Rheumatol. 42, 246–252 (2024).
Wang, H. et al. Mortality risk in patients with anti-MDA5 dermatomyositis is related to rapidly progressive interstitial lung disease and anti-Ro52 antibody. Arthritis Res. Ther. 25, 127 (2023).
Bhandari, S. et al. A review of MDA-5 dermatomyositis and associated interstitial lung disease. Rheumatology 4, 33–48 (2024).
Chen, X. et al. Clinical, radiological and pathological features of anti-MDA5 antibody-associated interstitial lung disease. RMD Open 9, e003150 (2023).
Shirai, T., Machiyama, T., Sato, H., Ishii, T. & Fujii, H. Intensive induction therapy combining tofacitinib, rituximab and plasma exchange in severe anti-melanoma differentiation-associatedprotein-5 antibody-positive dermatomyositis. Clin. Exp. Rheumatol. 41, 291–300 (2023).
Tsuji, H. et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 72, 488–498 (2020).
Nakashima, R. et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology 49, 433–440 (2009).
Koga, T. et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 51, 1278–1284 (2012).
Ishikawa, Y., Kasuya, T., Fujiwara, M. & Kita, Y. Tofacitinib for recurrence of antimelanoma differentiation-associated gene 5 antibody-positive clinically amyopathic dermatomyositis after remission: a case report. Medicine 99, e21943 (2020).
Endo, Y. et al. Recurrence of anti-MDA5 antibody-positive clinically amyopathic dermatomyositis after long-term remission. Medicine 97, e11024 (2018).
Lv, C. et al. Coexistence of anti-Ro52 antibodies in anti-MDA5 antibody-positive dermatomyositis is highly associated with rapidly progressive interstitial lung disease and mortality risk. J. Rheumatol. 50, 219–226 (2023).
Xu, A. et al. Prognostic values of anti-Ro52 antibodies in anti-MDA5-positive clinically amyopathic dermatomyositis associated with interstitial lung disease. Rheumatology 60, 3343–3351 (2021).
Allenbach, Y. et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology 95, e70–e78 (2020).
David, P. et al. MDA5-autoimmunity and interstitial pneumonitis contemporaneous with the COVID-19 pandemic (MIP-C). EBioMedicine 104, 105136 (2024).
Yamada, T. et al. RIG-I triggers a signaling-abortive anti-SARS-CoV-2 defense in human lung cells. Nat. Immunol. 22, 820–828 (2021).
Zou, J., Guo, Q., Chi, J., Wu, H. & Bao, C. HRCT score and serum ferritin level are factors associated to the 1-year mortality of acute interstitial lung disease in clinically amyopathic dermatomyositis patients. Clin. Rheumatol. 34, 707–714 (2015).
Gono, T. et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology 51, 1563–1570 (2012).
Nishioka, A. et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod. Rheumatol. 29, 814–820 (2019).
Liu, T. et al. Neutrophil-to-lymphocyte ratio is a predictive marker for anti-MDA5 positive dermatomyositis. BMC Pulm. Med. 22, 316 (2022).
Ren, F. P. et al. Characteristics and prognostic implications of peripheral blood lymphocyte subsets in patients with anti-MDA5 antibody positive dermatomyositis-interstitial lung disease. BMC Pulm. Med. 23, 411 (2023).
Zhang, P. et al. Plasma proteomic profiling reveals KRT19 could be a potential biomarker in patients with anti-MDA5+ dermatomyositis. Clin. Rheumatol. 42, 2145–2154 (2023).
Liu, Y. et al. IFN-beta and EIF2AK2 are potential biomarkers for interstitial lung disease in anti-MDA5 positive dermatomyositis. Rheumatology 62, 3724–3731 (2023).
Liu, Y. et al. Characteristics and predictors of malignancy in dermatomyositis: analysis of 239 patients from northern China. Oncol. Lett. 16, 5960–5968 (2018).
Kaji, K. et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology 46, 25–28 (2007).
Gunawardena, H. et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology 47, 324–328 (2008).
Satoh, M. et al. Autoantibodies to transcription intermediary factor (TIF)1β associated with dermatomyositis. Arthritis Res. Ther. 14, R79 (2012).
Mugii, N. et al. Oropharyngeal dysphagia in dermatomyositis: associations with clinical and laboratory features including autoantibodies. PLoS ONE 11, e0154746 (2016).
Rider, L. G. & Nistala, K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J. Intern. Med. 280, 24–38 (2016).
Halilu, F. & Christopher-Stine, L. Myositis-specific antibodies: overview and clinical utilization. Rheumatol. Immunol. Res. 3, 1–10 (2022).
Chung, M. P. et al. Calcinosis biomarkers in adult and juvenile dermatomyositis. Autoimmun. Rev. 19, 102533 (2020).
Valenzuela, A., Chung, L., Casciola-Rosen, L. & Fiorentino, D. Identification of clinical features and autoantibodies associated with calcinosis in dermatomyositis. JAMA Dermatol. 150, 724–729 (2014).
Fiorentino, D. F. et al. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J. Am. Acad. Dermatol. 72, 449–455 (2015).
Chinoy, H., Fertig, N., Oddis, C. V., Ollier, W. E. R. & Cooper, R. G. The diagnostic utility of myositis autoantibody testing for predicting the risk of cancer-associated myositis. Ann. Rheum. Dis. 66, 1345–1349 (2007).
Trallero-Araguás, E. et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 64, 523–532 (2012).
Cordel, N. et al. Anti-transcription intermediary factor 1-gamma IgG2 isotype is associated with cancer in adult dermatomyositis: an ENMC multinational study. Rheumatology 62, 1711–1715 (2023).
Hoshino, K. et al. Anti-MDA5 and anti-TIF1-γ antibodies have clinical significance for patients with dermatomyositis. Rheumatology 49, 1726–1733 (2010).
De Vooght, J. et al. Anti-TIF1-γautoantibodies: warning lights of a tumour autoantigen. Rheumatology 59, 469–477 (2020).
Kotobuki, Y., Tonomura, K. & Fujimoto, M. Transcriptional intermediary factor 1 (TIF1) and anti-TIF1γ antibody-positive dermatomyositis. Immunol. Med. 44, 23–29 (2020).
Pinal-Fernandez, I. et al. Tumour TIF1 mutations and loss of heterozygosity related to cancer-associated myositis. Rheumatology 57, 388–396 (2018).
Nguyen, H. D. et al. TIF1-gamma IgG2 isotype is not associated with malignancy in juvenile dermatomyositis patients. Rheumatology 63, e281–e284 (2024).
Adler, B. L. & Christopher-Stine, L. Triggers of inflammatory myopathy: insights into pathogenesis. Discov. Med. 25, 75–83 (2018).
Rothwell, S. et al. Focused HLA analysis in Caucasians with myositis identifies significant associations with autoantibody subgroups. Ann. Rheum. Dis. 78, 996–1002 (2019).
Oldroyd, A. G. S. et al. International guideline for idiopathic inflammatory myopathy-associated cancer screening: an international myositis assessment and clinical studies group (IMACS) initiative. Nat. Rev. Rheumatol. 19, 805–817 (2023).
Targoff, I. N., Trieu, E. P., Levy-Nato, M., Fertig, N. & Oddis, C. V. Sera with autoantibodies to the MJ antigen react with NXP2. Arthritis Rheum. 56, S787 (2007).
Mimura, Y., Takahashi, K., Kawata, K., Akazawa, T. & Inoue, N. Two-step colocalization of MORC3 with PML nuclear bodies. J. Cell Sci. 123, 2014–2024 (2010).
Oddis, C. V. Clinical and serological characterization of the anti-MJ antibody in childhood myositis. Arthritis Rheum. 40, S139 (1997).
Ichimura, Y. et al. Anti-nuclear matrix protein 2 antibody-positive inflammatory myopathies represent extensive myositis without dermatomyositis-specific rash. Rheumatology 61, 1222–1227 (2022).
Tansley, S. L. et al. Calcinosis in juvenile dermatomyositis is influenced by both anti-NXP2 autoantibody status and age at disease onset. Rheumatology 53, 2204–2208 (2014).
Albayda, J. et al. Antinuclear matrix protein 2 autoantibodies and edema, muscle disease, and malignancy risk in dermatomyositis patients. Arthritis Care Res. 69, 1771–1776 (2017).
Lundberg, I. E. et al. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Prim. 7, 86 (2021).
Xu, Y. et al. Gastrointestinal perforation in anti-NXP2 antibody-associated juvenile dermatomyositis: case reports and a review of the literature. Pediatr. Rheumatol. Online J. 19, 2 (2021).
Fu, Y. et al. Severe gastrointestinal involvements in patients with adult dermatomyositis with anti-NXP2 antibody. RMD Open 10, e003901 (2024).
Uchio, N. et al. Anti-nuclear matrix protein 2 antibody-positive dermatomyositis with gastrointestinal ulcers: a case report. Int. J. Rheum. Dis. 26, 2572–2575 (2023).
Wang, X. et al. Clinical characteristics and poor predictors of anti-NXP2 antibody-associated Chinese JDM children. Pediatr. Rheumatol. Online J. 19, 6 (2021).
Ichimura, Y. et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann. Rheum. Dis. 71, 710–713 (2012).
Landon-Cardinal, O. et al. Anti-Mi2 dermatomyositis revisited: pure DM phenotype with muscle fiber necrosis and high risk of malignancy. Neuromuscul. Disord. 27, S153 (2017).
Wolstencroft, P. W. & Fiorentino, D. F. Dermatomyositis clinical and pathological phenotypes associated with myositis-specific autoantibodies. Curr. Rheumatol. Rep. 20, 28 (2018).
Yasin, S. A. et al. Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: analysis by myositis autoantibody and pathological features. Neuropathol. Appl. Neurobiol. 45, 495–512 (2019).
Pinal-Fernandez, I. et al. More prominent muscle involvement in patients with dermatomyositis with anti-Mi2 autoantibodies. Neurology 93, e1768–e1777 (2019).
Fornaro, M. et al. Severe muscle damage with myofiber necrosis and macrophage infiltrates characterize anti-Mi2 positive dermatomyositis. Rheumatology 60, 2916–2926 (2021).
Liang, L. et al. Anti-Mi-2 antibodies characterize a distinct clinical subset of dermatomyositis with favourable prognosis. Eur. J. Dermatol. 30, 151–158 (2020).
Fujimoto, M. et al. Autoantibodies to small ubiquitin-like modifier activating enzymes in Japanese patients with dermatomyositis: comparison with a UK Caucasian cohort. Ann. Rheum. Dis. 72, 151–153 (2013).
Gono, T. et al. Two cases with autoantibodies to small ubiquitin-like modifier activating enzyme: a potential unique subset of dermatomyositis-associated interstitial lung disease. Int. J. Rheum. Dis. 22, 1582–1586 (2019).
Demortier, J. et al. Anti-SAE autoantibody in dermatomyositis: original comparative study and review of the literature. Rheumatology 62, 3932–3939 (2023).
Albayda, J. et al. A North American cohort of anti-SAE dermatomyositis: clinical phenotype, testing, and review of cases. ACR Open Rheumatol. 3, 287–294 (2021).
Ge, Y., Lu, X., Shu, X., Peng, Q. & Wang, G. Clinical characteristics of anti-SAE antibodies in Chinese patients with dermatomyositis in comparison with different patient cohorts. Sci. Rep. 7, 188 (2017).
Betteridge, Z. E. et al. Clinical and human leucocyte antigen class II haplotype associations of autoantibodies to small ubiquitin-like modifier enzyme, a dermatomyositis-specific autoantigen target, in UK Caucasian adult-onset myositis. Ann. Rheum. Dis. 68, 1621–1625 (2009).
Zuo, Y. et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology 59, 2829–2837 (2020).
Yang, H. et al. Identification of multiple cancer-associated myositis-specific autoantibodies in idiopathic inflammatory myopathies: a large longitudinal cohort study. Arthritis Res. Ther. 19, 259 (2017).
Pinal-Fernandez, I., Casal-Dominguez, M. & Mammen, A. L. Immune-mediated necrotizing myopathy. Curr. Rheumatol. Rep. 20, 21 (2018).
Wang, L. et al. Myopathy with anti-signal recognition particle antibodies: clinical and histopathological features in Chinese patients. Neuromuscul. Disord. 24, 335–341 (2014).
Svensson, J., Arkema, E. V., Lundberg, I. E. & Holmqvist, M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: a nationwide population-based study. Rheumatology 56, 802–810 (2017).
Dobloug, C. et al. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann. Rheum. Dis. 74, 1551–1556 (2015).
Kusumoto, T. et al. Development of necrotizing myopathy following interstitial lung disease with anti-signal recognition particle antibody. Intern. Med. 57, 2045–2049 (2018).
Ge, Y. et al. Interstitial lung disease is not rare in immune-mediated necrotizing myopathy with anti-signal recognition particle antibodies. BMC Pulm. Med. 22, 14 (2022).
Bandeira, M. et al. Predictors of cardiac involvement in idiopathic inflammatory myopathies. Front. Immunol. 14, 1146817 (2023).
Ma, X. & Bu, B. T. Anti-SRP immune-mediated necrotizing myopathy: a critical review of current concepts. Front. Immunol. 13, 1019972 (2022).
Allenbach, Y. et al. 224th ENMC International workshop: clinico-sero-pathological classification of immune-mediated necrotizing myopathies Zandvoort, The Netherlands, 14–16 October 2016. Neuromuscul. Disord. 28, 87–99 (2018).
Rouster-Stevens, K. A. & Pachman, L. M. Autoantibody to signal recognition particle in African American girls with juvenile polymyositis. J. Rheumatol. 35, 927–929 (2008).
Binns, E. L. et al. Effective induction therapy for anti-SRP associated myositis in childhood: a small case series and review of the literature. Pediatr. Rheumatol. Online J. 15, 77 (2017).
Pinal-Fernandez, I. et al. Longitudinal course of disease in a large cohort of myositis patients with autoantibodies recognizing the signal recognition particle. Arthritis Care Res. 69, 263–270 (2017).
Kurashige, T. Anti-HMGCR myopathy: clinical and histopathological features, and prognosis. Curr. Opin. Rheumatol. 33, 554–562 (2021).
Selva-O’Callaghan, A. et al. Statin-induced myalgia and myositis: an update on pathogenesis and clinical recommendations. Expert. Rev. Clin. Immunol. 14, 215–224 (2018).
Kishi, T. et al. Association of anti-3-hydroxy-3-methylglutaryl-coenzyme a reductase autoantibodies with DRB1*07:01 and severe myositis in juvenile myositis patients. Arthritis Care Res. 69, 1088–1094 (2017).
Khoo, T. & Chinoy, H. Anti-HMGCR immune-mediated necrotising myopathy: addressing the remaining issues. Autoimmun. Rev. 22, 103468 (2023).
Tiniakou, E. et al. More severe disease and slower recovery in younger patients with anti-3-hydroxy-3-methylglutarylcoenzyme A reductase-associated autoimmune myopathy. Rheumatology 56, 787–794 (2017).
Allenbach, Y. et al. High risk of cancer in autoimmune necrotizing myopathies: usefulness of myositis specific antibody. Brain 139, 2131–2135 (2016).
Connors, G. R., Christopher-Stine, L., Oddis, C. V. & Danoff, S. K. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138, 1464–1474 (2010).
Muro, Y. et al. Two novel anti-aminoacyl tRNA synthetase antibodies: autoantibodies against cysteinyl-tRNA synthetase and valyl-tRNA synthetase. Autoimmun. Rev. 21, 103204 (2022).
Preger, C. et al. Autoantigenic properties of the aminoacyl tRNA synthetase family in idiopathic inflammatory myopathies. J. Autoimmun. 134, 102951 (2023).
Vulsteke, J. B. et al. Mass spectrometry-based identification of new anti-Ly and known antisynthetase autoantibodies. Ann. Rheum. Dis. 82, 546–555 (2023).
Patel, P., Marinock, J. M., Ajmeri, A. & Brent, L. H. A review of antisynthetase syndrome-associated interstitial lung disease. Int. J. Mol. Sci. 21, 4453 (2024).
Satoh, M., Tanaka, S., Ceribelli, A., Calise, S. J. & Chan, E. K. L. A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin. Rev. Allergy Immunol. 52, 1–19 (2017).
Galindo-Feria, A. S., Wang, G. & Lundberg, I. E. Autoantibodies: pathogenic or epiphenomenon. Best. Pract. Res. Clin. Rheumatol. 36, 101767 (2022).
Marie, I. et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun. Rev. 11, 739–745 (2012).
Marie, I. et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur. J. Intern. Med. 24, 474–479 (2013).
Vulsteke, J. B. et al. Anti-OJ autoantibodies: rare or underdetected? Autoimmun. Rev. 18, 658–664 (2019).
Hamaguchi, Y. et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS ONE 8, e60442 (2013).
Kapoor, A., Vaidyan, P., Jalil, B. & Upaluri, C. Novel case of anti-synthetase syndrome. Eur. J. Rheumatol. 5, 275–277 (2018).
Noguchi, E. et al. Skeletal muscle involvement in antisynthetase syndrome. JAMA Neurol. 74, 992–999 (2017).
Wu, S. et al. Novel endotypes of antisynthetase syndrome identified independent of anti-aminoacyl transfer RNA synthetase antibody specificity that improve prognostic stratification. Ann. Rheum. Dis. 83, 775–786 (2024).
Aguila, L. A. et al. Clinical and laboratory features of overlap syndromes of idiopathic inflammatory myopathies associated with systemic lupus erythematosus, systemic sclerosis, or rheumatoid arthritis. Clin. Rheumatol. 33, 1093–1098 (2014).
Louis Gaspar, B. in Current Trends and Future Prospects Immune-Mediated Myopathies and Neuropathies 101–102 (Springer, 2023).
Srikantharajah, D., Lloyd, M. E. & Kiely, P. D. W. Rituximab and intravenous immunoglobulin treatment in PM/Scl antibody-associated disease: case-based review. Rheumatol. Int. 42, 359–364 (2022).
Jacquier, M. et al. Scleroderma renal crisis in a systemic sclerosis with anti-PM/Scl antibodies. Kidney Int. Rep. 4, 1499–1502 (2019).
Hanke, K. et al. Antibodies against PM/Scl-75 and PM/Scl-100 are independent markers for different subsets of systemic sclerosis patients. Arthritis Res. Ther. 11, R22 (2009).
Babu, A. K., Mizaj, Z., Thomas, J. & Chacko, M. Clinical significance of myositis-specific and myositis-associated antibody profiles in dermatomyositis. Indian. Dermatol. Online J. 14, 55–60 (2023).
Júnior, J. G., Mugii, N., Inaoka, P. T., Sampaio-Barros, P. D. & Shinjo, S. K. Inflammatory myopathies overlapping with systemic sclerosis: a systematic review. Clin. Rheumatol. 41, 1951–1963 (2022).
Fotis, L., Baszis, K. W., White, A. J. & French, A. R. Four cases of anti-PM/Scl antibody-positive juvenile overlap syndrome with features of myositis and systemic sclerosis. J. Rheumatol. 43, 1768–1769 (2016).
Rutkowska-Sak, L., Gietka, P., Gazda, A. & Kolodziejczyk, B. Juvenile systemic sclerosis — observations of one clinical centre. Reumatologia 59, 367–372 (2021).
Sharp, G. C., Irvin, W. S., Tan, E. M., Gould, R. G. & Holman, H. R. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am. J. Med. 52, 148–159 (1972).
Ciang, N. C. O., Pereira, N. & Isenberg, D. A. Mixed connective tissue disease—enigma variations? Rheumatology 56, 326–333 (2017).
Ge, Y. et al. Clinical characteristics of myositis patients with isolated anti-U1 ribonucleoprotein antibody resemble immune-mediated necrotizing myopathy. Ther. Adv. Musculoskelet. Dis. 15, 1759720–231181336 (2023).
Casal-Dominguez, M. et al. Muscular and extramuscular features of myositis patients with anti-U1-RNP autoantibodies. Neurology 92, e1416–e1426 (2019).
Lokesh, S., Tony, K., Raghupathy, Suresh, V. & Malepati, B. A rare case of mixed connective tissue disease (MCTD) with intricate features of lupus, polymyositis and rheumatoid arthritis presenting with severe myositis. J. Clin. Diagn. Res. 9, OD05–OD07 (2015).
Guha, S. et al. Exploring clinical features and therapeutic outcomes in Indian children with mixed connective tissue disease: a multicenter study. Int. J. Rheum. Dis. 27, e15243 (2024).
Wesner, N. et al. Anti-RNP antibodies delineate a subgroup of myositis: a systematic retrospective study on 46 patients. Autoimmun. Rev. 19, 102465 (2020).
Leclair, V. et al. Distinct HLA associations with autoantibody-defined subgroups in idiopathic inflammatory myopathies. EBioMedicine 96, 104804 (2023).
Ghirardello, A. et al. Detection of myositis autoantibodies by multi-analytic immunoassays in a large multicenter cohort of patients with definite idiopathic inflammatory myopathies. Diagnostics 13, 3080 (2023).
Pepper, E., Vilar, L. & Ward, I. M. Clinical characteristics and prognostic value of Ro52/SSA antibodies in idiopathic inflammatory myopathies. J. Clin. Rheumol. 29, 347–353 (2023).
Pina Cruellas, M. G. et al. Myositis-specific and myositis-associated autoantibody profiles and their clinical associations in a large series of patients with polymyositis and dermatomyositis. Clinics 68, 909–914 (2013).
Shi, J. et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J. Rheumatol. 44, 1051–1057 (2017).
Sreevilasan, S. K., Devarasetti, P., Narahari, N. K., Desai, A. & Rajasekhar, L. Clinical profile and treatment outcomes in antisynthetase syndrome: a tertiary centre experience. Rheumatol. Adv. Pract. 5, ii10–ii18 (2021). Suppl 2.
Limaye, V. S., Cassidy, J., Scott, G., Roberts-Thomson, P. J. & Gillis, D. Anti-Ro52 antibodies, antisynthetase antibodies, and antisynthetase syndrome. Clin. Rheumatol. 27, 521–523 (2008).
Yamasaki, Y. et al. Clinical subsets associated with different anti-aminoacyl transfer RNA synthetase antibodies and their association with coexisting anti-Ro52. Mod. Rheumatol. 26, 403–409 (2016).
Bauhammer, J. et al. Rituximab in the treatment of Jo1 antibody-associated antisynthetase syndrome: anti-Ro52 positivity as a marker for severity and treatment response. J. Rheumatol. 43, 1566–1574 (2016).
La Corte, R., Lo Mo Naco, A., Locaputo, A., Dolzani, F. & Trotta, F. In patients with antisynthetase syndrome the occurrence of anti-Ro/SSA antibodies causes a more severe interstitial lung disease. Autoimmunity 39, 249–253 (2006).
Shao, C. et al. Myositis specific antibodies are associated with isolated anti-Ro-52 associated interstitial lung disease. Rheumatology 61, 1083–1091 (2022).
Decker, P. et al. An updated review of anti-Ro52 (TRIM21) antibodies impact in connective tissue diseases clinical management. Autoimmun. Rev. 21, 103013 (2022).
Xia, J. et al. Respiratory symptoms as initial manifestations of interstitial lung disease in clinically amyopathic juvenile dermatomyositis: a case report with literature review. BMC Pediatr. 21, 488 (2021).
Liu, Y., Zheng, Y., Hao, H. & Yuan, Y. Narrative review of autoantibodies in idiopathic inflammatory myopathies. Ann. Transl. Med. 11, 291 (2023).
Zenone, T., Streichenberger, N. & Puget, M. Camptocormia as a clinical manifestation of polymyositis/systemic sclerosis overlap myositis associated with anti-Ku. Rheumatol. Int. 33, 2411–2415 (2013).
Spielmann, L. et al. Anti-Ku syndrome with elevated CK and anti-Ku syndrome with anti-dsDNA are two distinct entities with different outcomes. Ann. Rheum. Dis. 78, 1101–1106 (2019).
Sousa, M. et al. Anti-Ku antibody syndrome: is it a distinct clinical entity? A cross-sectional study of 75 patients. Rheumatology 62, e213–e215 (2023).
Casal-Dominguez, M. et al. The phenotype of myositis patients with anti-Ku autoantibodies. Semin. Arthritis Rheum. 51, 728–734 (2021).
Batu, E. D. et al. Further expanding the phenotype of anti-Ku antibody associated disease in children and adolescents. Neuromuscul. Disord. 40, 7–15 (2024).
Kanda, S. et al. Anti-Ku antibody-positive systemic sclerosis and idiopathic inflammatory myopathies overlap syndrome in children: a report of two cases and a review of the literature. Clin. Rheumatol. 42, 3411–3417 (2023).
Benjamin Larman, H. et al. Cytosolic 5′‐nucleotidase 1 A autoimmunity in sporadic inclusion body myositis. Ann. Neurol. 73, 408–418 (2013).
Pluk, H. et al. Autoantibodies to cytosolic 5′‐nucleotidase 1 A in inclusion body myositis. Ann. Neurol. 73, 397–407 (2013).
Lilleker, J. B. et al. 272nd ENMC international workshop: 10 years of progress — revision of the ENMC 2013 diagnostic criteria for inclusion body myositis and clinical trial readiness. 16–18 June 2023, Hoofddorp, The Netherlands.Neuromuscul. Disord. 37, 36–51 (2024).
Oldroyd, A., Lilleker, J. & Chinoy, H. Idiopathic inflammatory myopathies — a guide to subtypes, diagnostic approach and treatment. Clin. Med. 17, 322–328 (2017).
Salam, S., Dimachkie, M. M., Hanna, M. G. & Machado, P. M. Diagnostic and prognostic value of anti-cN1A antibodies in inclusion body myositis. Clin. Exp. Rheumatol. 40, 384–393 (2022).
Yeker, R. M. et al. Anti-NT5C1A autoantibodies are associated with more severe disease in patients with juvenile myositis. Ann. Rheum. Dis. 77, 714–719 (2018).
Rietveld, A. et al. Anti–cytosolic 5′- nucleotidase 1 A autoantibodies are absent in juvenile dermatomyositis. Arthritis Rheumatol. 73, 1329–1333 (2021).
Mammen, A. L., Pinal-Fernandez, I. & Rider, L. G. Conflicting reports of anti-cytosolic 5′-nucleotidase 1 A autoantibodies in juvenile dermatomyositis: comment on the article by Rietveld et al. Arthritis Rheumatol. 74, 911–912 (2022).
Herbert, M. K. et al. Disease specificity of autoantibodies to cytosolic 5′-nucleotidase 1 A in sporadic inclusion body myositis versus known autoimmune diseases. Ann. Rheum. Dis. 75, 696–701 (2016).
Kaji, K. et al. Autoantibodies to RuvBL1 and RuvBL2: a novel systemic sclerosis-related antibody associated with diffuse cutaneous and skeletal muscle involvement. Arthritis Care Res. 66, 575–584 (2014).
Di Pietro, L. et al. Anti-RuvBL1/2 autoantibodies detection in a patient with overlap systemic sclerosis and polymyositis. Antibodies 12, 13 (2023).
Landon-Cardinal, O. et al. Recognising the spectrum of scleromyositis: HEp-2 ANA patterns allow identification of a novel clinical subset with anti-SMN autoantibodies. RMD Open 6, e001357 (2020).
El Kamouni, H. et al. Anti-SMN autoantibodies in mixed connective tissue disease are associated with a severe systemic sclerosis phenotype. RMD Open 9, e003431 (2023).
Albrecht, I. et al. Development of autoantibodies against muscle-specific FHL1 in severe inflammatory myopathies. J. Clin. Invest. 125, 4612–4624 (2015).
Cowling, B. S. et al. Identification of FHL1 as a regulator of skeletal muscle mass: implications for human myopathy. J. Clin. Biol. 183, 1033–1048 (2008).
Galindo-Feria, A. S. et al. Autoantibodies against four-and-a-half-LIM domain 1 (FHL1) in inflammatory myopathies: results from an Australian single-centre cohort. Rheumatology 61, 4145–4154 (2022).
Sherman, M. A. et al. Anti-FHL1 autoantibodies in juvenile myositis are associated with anti-Ro52 autoantibodies but not with severe disease features. Rheumatology 62, S1226–S1234 (2023).
Fiorentino, D. F. et al. Immune responses to CCAR1 and other dermatomyositis autoantigens are associated with attenuated cancer emergence. J. Clin. Invest. 132, e150201 (2022).
Fiorentino, D. et al. Association of Anti-CCAR1 autoantibodies with decreased cancer risk relative to the general population in patients with anti-transcriptional intermediary factor 1γ-positive dermatomyositis. Arthritis Rheumatol. 75, 1238–1245 (2023).
Hosono, Y. et al. Coexisting autoantibodies against transcription factor Sp4 are associated with decreased cancer risk in patients with dermatomyositis with anti-TIF1γautoantibodies. Ann. Rheum. Dis. 82, 246–252 (2022).
Safe, S. MicroRNA-specificity protein (Sp) transcription factor interactions and significance in carcinogenesis. Curr. Pharmacol. Rep. 1, 73–78 (2015).
Sherman, M. A. et al. Autoantibodies recognizing specificity protein 4 co-occur with anti–transcription intermediary factor 1 and are associated with distinct clinical features and immunogenetic risk factors in juvenile myositis. Arthritis Rheumatol. 75, 1668–1677 (2023).
Labrador-Horrillo, M. et al. Identification of a novel myositis-associated antibody directed against cortactin. Autoimmun. Rev. 13, 1008–1012 (2014).
Pinal-Fernandez, I. et al. Anti-cortactin autoantibodies are associated with key clinical features in adult myositis but are rarely present in juvenile myositis. Arthritis Rheumatol. 74, 358–364 (2021).
Buday, L., & Downward, J. Roles of cortactin in tumor pathogenesis. Biochim. Biophys. Acta 263-273, 2007 (1775).
Choi, M. Y., Satoh, M. & Fritzler, M. J. Update on autoantibodies and related biomarkers in autoimmune inflammatory myopathies. Curr. Opin. Rheumatol. 35, 383–394 (2023).
Nagai, A. et al. Clinical features of anti-mitochondrial M2 antibody-positive myositis: case series of 17 patients. J. Neurol. Sci. 442, 120391 (2022).
Maeda, M. H., Tsuji, S. & Shimizu, J. Inflammatory myopathies associated with anti-mitochondrial antibodies. Brain 135, 1767–1777 (2012).
Uhl, G. S., Baldwin, J. L. & Arnett, F. C. Primary biliary cirrhosis in systemic sclerosis (scleroderma) and polymyositis. Johns. Hopkins Med. J. 135, 191–198 (1974).
Fujii, S. et al. Inflammatory myopathy associated with anti-mitochondrial antibody presenting only with respiratory failure. Intern. Med. 60, 3801–3804 (2021).
Albayda, J. et al. Inflammatory myopathy associated with anti-mitochondrial antibodies: a distinct phenotype with cardiac involvement. Semin. Arthritis Rheum. 47, 552–556 (2018).
Minamiyama, S. et al. Thigh muscle MRI findings in myopathy associated with anti-mitochondrial antibody. Muscle Nerve 61, 81–87 (2020).
Ishizuka, K. & Ohira, Y. Antimitochondrial antibody-positive myositis. Am. J. Med. 137, e38–e39 (2024).
Betteridge, Z. et al. Identification of a novel autoantigen eukaryotic initiation factor 3 associated with polymyositis. Rheumatology 59, 1026–1030 (2020).
La Rocca, G. et al. Targeting intracellular pathways in idiopathic inflammatory myopathies: a narrative review. Front. Med. 10, 1158768 (2023).
Gupta, L. & Chinoy, H. Monitoring disease activity and damage in adult and juvenile idiopathic inflammatory myopathy. Curr. Opin. Rheumatol. 32, 553–561 (2020).
do Vale Pascoal Rodrigues, P. R. et al. Triple-seronegative myasthenia gravis: clinical and epidemiological characteristics. Arq. Neuropsiquiatr. 82, 1–7 (2024).
Wigerblad, G. et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine-dependent mechanism. Ann. Rheum. Dis. 75, 730–738 (2016).
Catrina, A., Krishnamurthy, A. & Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 7, e001228 (2021).
Sakamoto, S. et al. Low positive titer of anti-melanoma differentiation-associated gene 5 antibody is not associated with a poor long-term outcome of interstitial lung disease in patients with dermatomyositis. Respir. Investig. 56, 464–472 (2018).
Cao, H. et al. Clinical manifestations of dermatomyositis and clinically amyopathic dermatomyositis patients with positive expression of anti-melanoma differentiation-associated gene 5 antibody. Arthritis Care Res. 64, 1602–1610 (2012).
Matsushita, T. et al. Antimelanoma differentiation-associated protein 5 antibody level is a novel tool for monitoring disease activity in rapidly progressive interstitial lung disease with dermatomyositis. Br. J. Dermatol. 176, 395–402 (2017).
Muro, Y., Sugiura, K., Hoshino, K. & Akiyama, M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology 51, 800–804 (2012).
Tiniakou, E. et al. Anti-MDA5-positive dermatomyositis and remission in a single referral centre population. Clin. Exp. Rheumatol. 41, 309–315 (2023).
Hall, J. C. et al. Anti-melanoma differentiation-associated protein 5-associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 65, 1307–1315 (2013).
Chen, B.-H., Zhu, X.-M., Xie, L. & Hu, H.-Q. Immune-mediated necrotizing myopathy: report of two cases. World J. Clin. Cases. 11, 3552–3559 (2023).
Benveniste, O. et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 63, 1961–1971 (2011).
Liu, R. et al. Pathogenic role and clinical significance of neutrophils and neutrophil extracellular traps in idiopathic inflammatory myopathies. Clin. Exp. Med. 24, 115 (2024).
Bolko, L. et al. The role of interferons type I, II and III in myositis: a review. Brain Pathol. 31, e12955 (2021).
Peng, Y., Zhang, S., Zhao, Y., Liu, Y. & Yan, B. Neutrophil extracellular traps may contribute to interstitial lung disease associated with anti-MDA5 autoantibody positive dermatomyositis. Clin. Rheumatol. 37, 107–115 (2018).
Seto, N. et al. Neutrophil dysregulation is pathogenic in idiopathic inflammatory myopathies. JCI Insight 5, e134189 (2020).
Zhao, L. et al. Machine learning algorithms identify clinical subtypes and cancer in anti-TIF1γ+ myositis: a longitudinal study of 87 patients. Front. Immunol. 13, 802499 (2022).
Zhang, S. et al. Enhanced formation and impaired degradation of neutrophil extracellular traps in dermatomyositis and polymyositis: a potential contributor to interstitial lung disease complications. Clin. Exp. Immunol. 177, 134–141 (2014).
Arouche-Delaperche, L. et al. Pathogenic role of anti-signal recognition protein and anti-3-Hydroxy-3-methylglutaryl-CoA reductase antibodies in necrotizing myopathies: myofiber atrophy and impairment of muscle regeneration in necrotizing autoimmune myopathies. Ann. Neurol. 81, 538–548 (2017).
Bergua, C. et al. In vivo pathogenicity of IgG from patients with anti-SRP or anti-HMGCR autoantibodies in immune-mediated necrotising myopathy. Ann. Rheum. Dis. 78, 131–139 (2019).
Nishikai, M. & Reichlin, M. Heterogeneity of precipitating antibodies in polymyositis and dermatomyositis. Characterization of the Jo-1 antibody system. Arthritis Rheum. 23, 881–888 (1980).
Mathews, M. B. & Bernstein, R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature 304, 177–179 (1983).
Mahler, M. et al. Comparison of three immunoassays for the detection of myositis specific antibodies. Front. Immunol. 10, 848 (2019).
Damoiseaux, J., Mammen, A. L., Piette, Y., Benveniste, O. & Allenbach, Y. 256th ENMC international workshop: myositis specific and associated autoantibodies (MSA-ab): Amsterdam, The Netherlands, 8–10 October 2021. Neuromuscul. Disord. 32, 594–608 (2022).
Loganathan, A. et al. The use of ELISA is comparable to immunoprecipitation in the detection of selected myositis-specific autoantibodies in a European population. Front. Immunol. 13, 975939 (2022).
Ghirardello, A. et al. Diagnostic performance and validation of autoantibody testing in myositis by a commercial line blot assay. Rheumatology 49, 2370–2374 (2010).
Tansley, S. L. et al. The promise, perceptions, and pitfalls of immunoassays for autoantibody testing in myositis. Arthritis Res. Ther. 22, 117 (2020).
Cavazzana, I. et al. Evaluation of a novel particle-based assay for detection of autoantibodies in idiopathic inflammatory myopathies. J. Immunol. Methods 474, 112661 (2019).
Mahler, M. & Fritzler, M. J. Detection of myositis-specific antibodies: additional notes. Ann. Rheum. Dis. 78, e29 (2019).
Espinosa-Ortega, F. et al. Comparison of autoantibody specificities tested by a line blot assay and immunoprecipitation-based algorithm in patients with idiopathic inflammatory myopathies. Ann. Rheum. Dis. 78, 858–860 (2019).
Tansley, S. L., Li, D., Betteridge, Z. E. & McHugh, N. J. The reliability of immunoassays to detect autoantibodies in patients with myositis is dependent on autoantibody specificity. Rheumatology 59, 2109–2114 (2020).
Anderson, H. T., O’Donnell, J. L., Tustin, P. & Steele, R. Diagnosis and subtyping of idiopathic inflammatory myopathies: caution required in the use of myositis autoantibodies. Intern. Med. J. 54, 682–686 (2024).
Chang, Y. C., Yang, L. & Budhram, A. Positive predictive value of myositis antibody line blot testing in patients with suspected idiopathic inflammatory myopathy. Muscle Nerve 69, 626–630 (2024).
Cavazzana, I. et al. Testing for myositis specific autoantibodies: comparison between line blot and immunoprecipitation assays in 57 myositis sera. J. Immunol. Methods 433, 1–5 (2016).
Nakashima, R. et al. The multicenter study of a new assay for simultaneous detection of multiple anti-aminoacyl-tRNA synthetases in myositis and interstitial pneumonia. PLoS ONE 9, e85062 (2014).
Sato, S. et al. Clinical utility of an enzyme-linked immunosorbent assay for detecting anti-melanoma differentiation-associated gene 5 autoantibodies. PLoS ONE 11, e0154285 (2016).
Muro, Y., Sugiura, K. & Akiyama, M. A new ELISA for dermatomyositis autoantibodies: rapid introduction of autoantigen cDNA to recombinant assays for autoantibody measurement. Clin. Dev. Immunol. 2013, 856815 (2013).
Waritani, T., Chang, J., McKinney, B. & Terato, K. An ELISA protocol to improve the accuracy and reliability of serological antibody assays. MethodsX 4, 153–165 (2017).
Bundell, C., Rojana-Udomsart, A., Mastaglia, F., Hollingsworth, P. & McLean-Tooke, A. Diagnostic performance of a commercial immunoblot assay for myositis antibody testing. Pathology 48, 363–366 (2016).
Vulsteke, J.-B. et al. Detection of myositis-specific antibodies. Ann. Rheum. Dis. 78, e7 (2019).
Richards, M. et al. Autoantibodies to Mi-2 alpha and Mi-2 beta in patients with idiopathic inflammatory myopathy. Rheumatology 58, 1655–1661 (2019).
Lundberg, I. E. et al. 2017 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Adult and Juvenile Idiopathic Inflammatory Myopathies and Their Major Subgroups. Arthritis Rheumatol. 69, 2271–2282 (2017).
Acknowledgements
L.R.W. is supported by the National Institute for Health Research (NIHR) via the NIHR-Biomedical Research Centre at Great Ormond Street Hospital and an NIHR Senior Investigator award. The views expressed are those of the author and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
D.A.I., N.A.A. and A.I.R.-L. researched data for the article and contributed substantially to discussion of the content. All authors wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nature Reviews Rheumatology thanks Chester Oddis and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Allameen, N.A., Ramos-Lisbona, A.I., Wedderburn, L.R. et al. An update on autoantibodies in the idiopathic inflammatory myopathies. Nat Rev Rheumatol 21, 46–62 (2025). https://doi.org/10.1038/s41584-024-01188-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-024-01188-4