Thanks to modern healthcare and improved living standards, life expectancy is predicted to surpass 80 years in most parts of the world by 2050. That’s the positive.
The negative is that will push the number of people in the world over the age of 60 to more than 2 billion, which in turn will make age-related health issues a tremendous global burden for which we’re deeply unprepared.
There will be many knock-on effects on the world’s health-care and social welfare systems, but we’ve also made significant progress on many of the illnesses more likely to afflict the elderly, including cancer and heart disease. One we have not done much for also happens to be one of the most tragic forms of aging gone awry: dementia, the umbrella term for the symptoms some older adults experience as they’re slowly robbed of their sense of self and cognitive abilities.
Dementia isn’t a disease itself, but is caused by many. In 60% to 80% of dementia cases, the cause is Alzheimer’s disease. In the US, where older adults are on the verge of outnumbering children for the first time ever, a new case of Alzheimer’s is now diagnosed every 66 seconds. This year, the total cost of caring for all of the people in the US with this disease is expected to reach $1 trillion—higher than it’s ever been before. And yet despite what has obviously become a crisis, there hasn’t been a new treatment for Alzheimer’s in over a decade.
🧠 🧠 🧠
There are fairly effective psychiatric medication options for the anxiety that comes with living with constant confusion, and there are currently five drugs on the market that can slow Alzheimer’s-related memory loss. But none are great, and the most recent memory loss-prevention drug to make it to market was 15 years ago: memantine, manufactured by Forest Labs under the brand name Namenda, was approved by the US Food and Drug Administration (FDA) in 2003.
Since then, the stories of disappointing clinical trials have become almost mundane. Earlier this year, Eli Lilly announced that a phase III clinical trial for an Alzheimer’s drug to clear amyloid plaques from the brain had failed (paywall). They had been testing the compound in people with various stages of the disease since 2012. In the past three years, Axovant, Merck, Biogen, and Prana Biotech all reported similar failures of compounds in their pipelines that had looked promising—and had the potential to earn the companies billions of dollars. Pfizer’s own setbacks in clinical testing caused it to drop out of the Alzheimer’s drug game entirely in January 2018.
There are three main reasons scientists have not successfully developed new Alzheimer’s drugs in the last decade and a half. The first is a complication of the disease itself: for years, it’s asymptomatic. Neurological symptoms only begin to show when the disease has progressed to the point where it has irreversibly damaged the brain, at which point it’s almost impossible to treat.
The logical work-around would be to start treating people sooner. But the second roadblock is that there are no good methods to tell if someone has the early, biological stages of the disease. At the moment, the only definitive diagnostic tools for Alzheimer’s detection are costly or painful, and are therefore only used when doctors strongly suspect their patients have Alzheimer’s or another form of dementia based on their symptoms.
The problem of identifying the disease leads to the third hurdle: finding candidates for effective clinical trials. Ideally, trials would include a cohort of people who may develop Alzheimer’s, with some receiving an intervention and others not; the efficacy of a given treatment would then be assessed by looking at what percentage of of each group got sick. Healthy people, though, have no incentive to enroll in these types of drug studies, and there’s no good way to identify people with risk for the disease, or even initial biological signs of the disease. In essence, scientists currently have no way to study Alzheimer’s patients for long enough to test memory-loss prevention drugs.
Although Alzheimer’s research has made major strides in the past century, the disease is like a hydra: for every question answered, two more appear.
There’s a potential solution that knocks out all three problems at once: a better way of detecting the first signs of Alzheimer’s. Scientists today understand the path ahead of them, and have the financial support needed to carry on. It’s now a race; we need breakthroughs as soon as possible to provide the right interventions for all of us, since we’re all growing older every day.
🧠 🧠 🧠
Although Alzheimer’s disease was first documented in the beginning of the 20th century, the illness didn’t receive mainstream medical attention until about 40 years ago. The largest Alzheimer’s advocacy group in the world was started by a container-company CEO named Jerome Stone. After his wife was diagnosed with Alzheimer’s in 1970, Stone recognized there was no national organization to support the disease. For nine years, he worked to unite seven smaller groups that had formed independently across the US, eventually establishing the Alzheimer’s Association, headquartered in Chicago, Illinois.
Since then, the Alzheimer’s Association has successfully organized chapters throughout the US, which in turn have lobbied Congress year after year for research funding. In 2012, they hit their first major milestone when then-president Barack Obama signed the National Alzheimer’s Project Act which created a national plan to end Alzheimer’s disease by 2025, and, more practically, allotted millions of non-negotiable federal dollars every year to fund Alzheimer’s research.
The National Alzheimer’s Project Act spurred the Alzheimer’s Accountability Act to be put into law starting in the fiscal year of 2015. This law allows (pdf) federal funding for Alzheimer’s research to sidestep the annual budget-approval process. Each year, the US National Institutes of Health (NIH) prepares a report for the president and Congress outlining its funding needs in order to meet the 2025 target of curing of preventing the disease. As long Congress approves this request and the president signs off on it, this funding request is solidified and non-negotiable as part of the federal budget. Alzheimer’s, HIV, and cancer are the only illnesses funded in this unconditional way.
Indeed, the NIH more than doubled research funding set aside strictly for Alzheimer’s disease recently, from $631 million in 2015 to $1.4 billion in 2017. This year, Alzheimer’s research funding was raised even further, to an estimated $1.9 billion.
Robert Egge, the chief public policy officer at the Alzheimer’s Association, says that research lags behind funding. “We’re still basically dealing with science in terms of results that were funded before the ramp up” in federal research grants, he says.
Scientists seem to find new complications to the disease every day. “It seems,” says Ursula Staudinger, a psychologist and aging researcher at Columbia University, “that the molecular biology [of Alzheimer’s] is just starting to be understood.”
🧠 🧠 🧠
Alois Alzheimer, a German psychiatrist working around the turn of the 20th century, was the first to notice that there’s more than one type of dementia. He’d treated numerous dementia patients in his career, in 1906, when he came across a strange case: a woman who appeared to have died at age 55 (paywall) because of advanced dementia. It was strange because he’d never seen anyone develop the illness or die from it at such an early age.
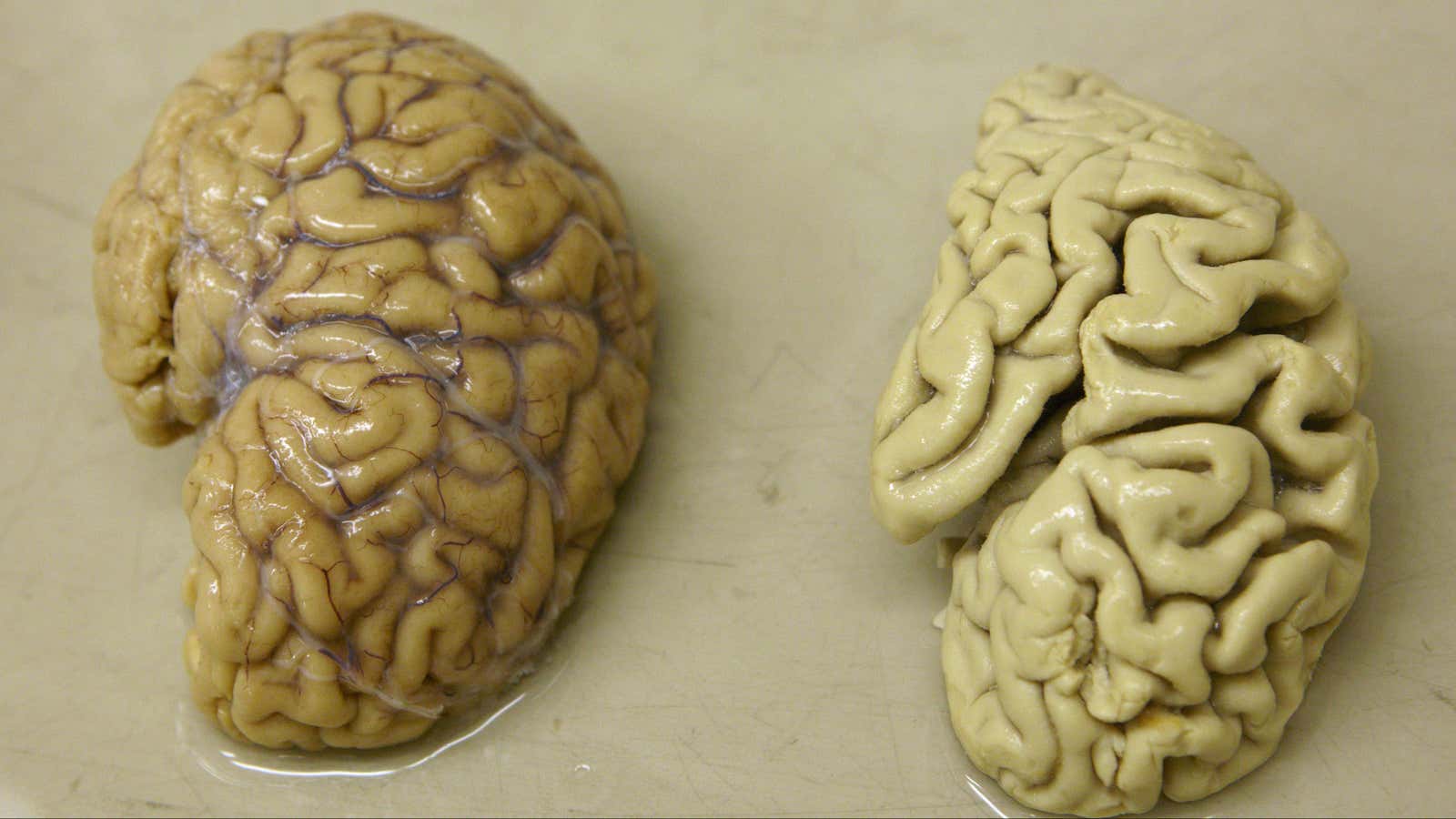
While conducting an autopsy on the woman a few years later, Alzheimer discovered that her brain looked unlike the brains of any other patients with dementia he’d examined post-mortem in the past. This woman’s brain had shriveled and was peppered with clumps of hard plaques and knotted fibers.
Four years later, another German, a psychiatrist named Emil Kraepelin wrote about the case study in the journal General Psychiatry. Soon, physicians began to recognize the disease in cases across Europe and North America, and started to call it Alzheimer’s, after Alois. And yet, for decades after its initial documentation, the disease remained a neurological mystery.
Doctors and researchers did start to learn some things about Alzheimer’s. For example, through post-mortem analyses, they realized that, for some reason, Alzheimer’s patients had unusual buildups of proteins called amyloids in their brains. These proteins are a normal part of everyday function in healthy people, but instead of breaking down as they should, they form plaques that eventually destroy neurons.
Simultaneously—although it’s unclear if this is because of the amyloid buildups or in addition to them—proteins called tau, which form connections within and between neurons, become dense with extra phosphorus atoms. Eventually, the tau proteins become knotted, and end up destroying affected neurons, which also impairs the connection between other neurons. Worse, the tau then starts spreading. The destruction starts in the hippocampus, the tiny, seahorse-shaped memory hub at the base of the center of the brain, and extends outward, eventually overwhelming the entire organ.
For a while, the brain maintains normal function with these malformed proteins and dying neurons, but eventually, it hits a tipping point. The hippocampus starts to shrink and people who could once remember to-do lists, names, and directions start to become more forgetful and confused, which understandably leads to intense anxiety and confusion. The cognitive symptoms only get worse from there, followed by physical decline that is eventually fatal.
The damage caused by the disease is permanent. Even healthy adult brains don’t seem to be able to grow new neurons. Older, weakened brains don’t stand a chance.
🧠 🧠 🧠
In theory, the best way to treat Alzheimer’s disease would be to stop the brain-cell destruction before it starts. The problem is that during the earliest stages of Alzheimer’s, people have no way of knowing that anything is wrong. For all their capabilities, our brains don’t feel anything; it’s impossible for us to tell when the organ itself is damaged. That’s why, usually, when cognitive symptoms do show up, it’s too late. The neurons have already died after being besieged by their warped environment for 10 to 20 years.
So even if we somehow came up with a treatment that stopped the disease before symptoms started, we’d need to come up with a way to actually identify patients with early amyloid build ups.
Even definitively diagnosing Alzheimer’s disease is a relatively new medical feat. For a long time, the only way to confirm a case was through autopsies. For their living patients, doctors relied on indirect measures of the disease, says James Hendrix, the director of Global Science Initiatives, Medical and Scientific Relations at the Alzheimer’s Association. Brain-imaging technology to rule out tumors or check the size of the hippocampus and surrounding brain regions, but being inconclusive, were not ideal tools.
Doctors are trained to think “horse” when they hear the sound of hooves, not “zebra.” When an older patient begins to complain about memory problems, it makes sense to assume it’s related to Alzheimer’s given that the disease makes up the majority cases of dementia. The problem, though, is that incorrectly diagnosed Alzheimer’s patients could end up taking medication that harms them.
For example, the first drug approved in the US to treat Alzheimer’s, a compound called tacrine and sold under the brand name Cognex, caused such severe liver damage that it was taken off the market in 2013. While tacrine may have helped some small number of patients who actually had Alzheimer’s, it also came with severe side effects. Anyone who was erroneously diagnosed with Alzheimer’s would have encountered those same side effects without the same benefit.
There have been some upgrades to the diagnostic toolkit for Alzheimer’s in recent years. In 2004, researchers from the University of Pittsburgh showed (paywall) that positron emission tomography (PET) scans can detect amyloid buildup in the brains of Alzheimer’s patients. Around the same time, researchers at the NIH discovered (paywall) amyloid-forming proteins can be isolated from the fluid that surrounds the brain from a spinal tap. Practically, though, these tests are invasive (in the case of the spinal tap) and expensive (in the case of a PET scan, which can go for around $3,000 without insurance).
Scientists are now working on blood tests that can detect the presence of early amyloid plaques in the brain. These, Morris says, are are in an “infancy” stage, and none have been approved for clinical use. But when—if—they do prove ready for showtime, they could reverse the recent, bleak trends in Alzheimer’s drug research.
🧠 🧠 🧠
Starting in the early 2000s, Darrell Foss sensed something was wrong. It was a subtle change: Fixing things around the house became difficult, names of friends slipped his mind, and he couldn’t remember how to cut his wife’s hair, which he’d been doing since they first married. But he ignored it.
Roughly a decade later in 2015, Foss was making bacon for his family at their home in Eagan, Minnesota. He took the hot grease from the pan and, instead of pouring it into an old glass jar like he usually would, he sloshed it all over the counter. “This is just what we do,” he said calmly to his bewildered family.
At his wife’s urging, Foss finally visited his doctor, who diagnosed him with mild cognitive impairment—often the first step on a path to Alzheimer’s. At the time, he was 71 years old. “I actually went into a period of depression,” Foss says. “The name creates all kinds of these really terrible, awful thoughts that go through your mind. Like, ‘I’m gonna become a vegetable, I’m going to die from this.’”
His wife Mary, however, didn’t let him wallow. She motivated him to get involved in the North Dakota and Minnesota chapter of the Alzheimer’s Association. That eventually led Foss to workshops that taught him how to better live with his condition—and got him into three clinical trials for experimental treatments.
Foss was lucky to find these groups, but they were just as lucky to find him. For a long time, recruiting patients for these trials has been an extra headache for researchers.
At the moment, most researchers rely on patient networks to find eligible people for clinical trials. Perhaps the largest is the Dominantly Inherited Alzheimer Network, created by Morris and his colleagues at Washington University-St. Louis. This network is made up of people with one of three genetic mutations that essentially guarantees they will develop signs of Alzheimer’s in their 40s or 50s. Dominantly Inherited Alzheimer’s makes up about 1% of all Alzheimer’s cases.
In an ideal world, this group of roughly 57,000 living in the US would be identified and could participate in some kind of research study. After all, these people are ideal candidates for early trials because testing on them gives researchers at least some sense of whether or not a particular drug or other intervention has worked. But people to opt in to specifically get tested for these Alzheimer’s mutations, and opt in again to participate in these trials. A key component of medical research is that it is voluntary, and would be wrong to force them to do either.
Then there are the other networks of Alzheimer’s patients who would have been good candidates for drug trials—except, by the time they were diagnosed, it was too late for them to be ideal candidates for these trials. For the most part, these are people who sought help for early stages of dementia—except the thinking now is that when Alzheimer’s is recognizable, it means it’s passed the point of treatment. “All of those failed trials were enrolling individuals who already had the symptoms of Alzheimer’s disease,” says Morris.
The NIH recognizes this problem. Late last year, the agency announced a program to streamline clinical trials for Alzheimer’s by combining all the known databases of possible candidates for Alzheimer’s drugs. That should mean trials can get participants more easily, and at earlier stages of the disease’s progression.
That’s essential because many of the studies to date have involved patients too far down the line well past the optimal window of intervention, which means a drug may appear to work based on biomarkers, but still not show measurable improvements in patients’ symptoms. Without the latter, the drug can’t get FDA approval.
In addition, Alzheimer’s symptoms show up differently in each case. “Mild cognitive impairment,” usually the first step in a diagnosis, can mean anything from forgetting friends’ names to getting lost on familiar routes to being unable to make plans for the day. It’s nearly impossible to show with any certainty that a potential new drug works comparably across a number of people with a number of different symptoms.
This year, the FDA took some of the first steps to change the way it assesses clinical trials for Alzheimer’s drugs. In a draft report released in March, the agency said it would accept a decline in biomarkers for Alzheimer’s disease—presumably meaning smaller amounts of amyloid or tau buildup—as enough evidence that a particular treatment works. If this framework were to be made law, for a drug did make it to market, its manufacturing company would be required to look for changes in cognition or daily function among those taking the medication. These guidelines aren’t finalized, but many researchers in the field support them.
In any case, these changes are not likely to bring in more eligible participants. Like Foss, many people who will go on to have the disease often start off in denial about their neurological symptoms. Even if they do make it to their healthcare provider for a visit, they may not be diagnosed accurately. A 2014 review by NIH researchers found that doctors rarely evaluate patients’ cognition thoroughly enough to make an Alzheimer’s diagnosis because of time pressures.
And even if a doctor does diagnose his or her patient with early-stage Alzheimer’s, other factors may exclude that patient from clinical studies. Disqualifying factors include high blood pressure, other vascular diseases, and not having a partner. That’s right: most studies require that participants have a romantic partner in their lives who can comment on their day-to-day function. Potential participants who live alone—those who arguably need to be in these studies the most—are often excluded.
🧠 🧠 🧠
Improving diagnostics is merely a battle in the fight to end Alzheimer’s. To truly win the war, scientists would have to identify what causes the disease in the first place to prevent it.
There are a number of working theories. Some experts think it’s a result of diabetes or other vascular diseases. Others believe the immune system is at fault. Although both theories are promising, right now they’re based just on statistical correlations revealed in cohort studies. Scientists have yet prove these conditions actually cause degenerative brain disease.
In the meantime, scientists say the best use of research funds is to keep digging into the promising field of lifestyle-intervention. Last year, the National Academies of Sciences published what it calls an “inconclusive but encouraging” report on the merits of diet, exercise, and social interaction when it comes to Alzheimer’s prevention. For example, small studies in humans and mice have shown that exercise can reduce the risk for developing Alzheimer’s. Other longitudinal studies have suggested that social interaction can stave off dementia. And there seems to be a link between diets rich in vitamins E and omega-3 fatty acids and decreased risk for developing Alzheimer’s, whereas diets rich in processed food seem to increase these risks.
But it remains unclear why any of these interventions work, and it’s unlikely we’ll figure out why any time soon. Though government funding for Alzheimer’s research has increased in recent years, pharmaceutical companies still have the deepest pockets. It may cost billions of dollars to run a clinical trial, but drug companies can usually count on future sales to make up for it (and then some). You can’t patent exercise or salmon-rich diets, though. Without the promise of a big payoff, it’s doubtful pharmaceutical companies will fund studies to explore whether and how these lifestyle interventions work.
The good news, though, is unlike a new drug, going for more walks, joining a bridge club, or cutting down on foods high in trans fats all carry no real health risk. All of these lifestyle choices would be good for anyone (unless you really hate bridge). The problem is that they just may not be enough.
🧠 🧠 🧠
The outlook for Alzheimer’s treatment depends a lot on your perspective. The next steps of Alzheimer’s research will have to follow large, diverse populations to pinpoint the nuances of the disease’s progression to find exactly when—and how—doctors can intervene. Science, especially when it necessitates clinical trials that follow patients for years, takes a long time to yield results.
But in this case, we don’t have a lot of time. Scientists have seven years to meet the 2025 goal set out by the government’s National Alzheimer’s Project Act. In clinical research, especially following a progressive disease, seven years is a blink of an eye. And the rate at which people are diagnosed with Alzheimer’s will almost certainly increase in the coming years as the global population continues to age. Increased life expectancy means increased chances of living with the disease; almost 40% of those older than 85 have Alzheimer’s.
Past pharmaceutical failures may be discouraging, but in some sense they’ve been important, says Egge. They’ve demonstrated how complex Alzheimer’s disease really is, which encourages more funding from groups like the NIH. Knowing what doesn’t work and how even the structure of research needs to be improved, has been crucial for laying out the path forward.
This article has been updated to reflect the most recent NIH estimates for Alzheimer’s and dementia research funding in 2018.
Correction: This article has been updated to clarify that Congress has to approve federal funding for Alzheimer’s research before the president signs off on it.