Abstract
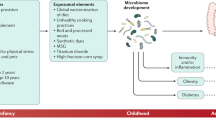
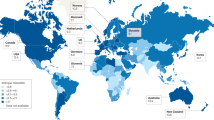
The incidence of early-onset colorectal cancer (CRC), which occurs in individuals <50 years of age, has been increasing worldwide and particularly in high-income countries. The reasons for this increase remain unknown but plausible hypotheses include greater exposure to potential risk factors, such as a Western-style diet, obesity, physical inactivity and antibiotic use, especially during the early prenatal to adolescent periods of life. These exposures can not only cause genetic and epigenetic alterations in colorectal epithelial cells but also affect the gut microbiota and host immunity. Early-onset CRCs have differential clinical, pathological and molecular features compared with later-onset CRCs. Certain existing resources can be utilized to elucidate the aetiology of early-onset CRC and inform the development of effective prevention, early detection and therapeutic strategies; however, additional life-course cohort studies spanning childhood and young adulthood, integrated with prospective biospecimen collections, omics biomarker analyses and a molecular pathological epidemiology approach, are needed to better understand and manage this disease entity. In this Perspective, we summarize our current understanding of early-onset CRC and discuss how we should strategize future research to improve its prevention and clinical management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Arnold, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut 66, 683–691 (2017).
Global Burden of Disease Cancer Collaboration et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 3, 524–548 (2017).
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394, 1467–1480 (2019).
Keum, N. & Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732 (2019).
Siegel, R. L. et al. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 70, 145–164 (2020).
Siegel, R. L. et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut 68, 2179–2185 (2019).
Vuik, F. E. et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 68, 1820–1826 (2019).
Lui, R. N. et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: a joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol. Biomarkers Prev. 28, 1275–1282 (2019).
Saad El Din, K. et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer 20, 288 (2020).
Meester, R. G. S., Mannalithara, A., Lansdorp-Vogelaar, I. & Ladabaum, U. Trends in incidence and stage at diagnosis of colorectal cancer in adults aged 40 through 49 years, 1975–2015. JAMA 321, 1933–1934 (2019).
Siegel, R. L. et al. Colorectal cancer incidence patterns in the United States, 1974–2013. J. Natl Cancer Inst. 109, djw322 (2017).
Chung, R. Y. et al. A population-based age-period-cohort study of colorectal cancer incidence comparing Asia against the West. Cancer Epidemiol. 59, 29–36 (2019).
Brenner, D. R. et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev. Med. 105, 345–349 (2017).
Feletto, E. et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: analysis of data on over 375,000 cases. Cancer Epidemiol. Biomarkers Prev. 28, 83–90 (2019).
Stoffel, E. M. & Murphy, C. C. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology 158, 341–353 (2020).
Sung, J. J. Y. et al. Increasing trend in young-onset colorectal cancer in Asia: more cancers in men and more rectal cancers. Am. J. Gastroenterol. 114, 322–329 (2019).
Sung, H., Siegel, R. L., Rosenberg, P. S. & Jemal, A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health 4, e137–e147 (2019).
Gupta, S. et al. International trends in the incidence of cancer among adolescents and young adults. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa007 (2020).
Rosato, V. et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 24, 335–341 (2013).
Gausman, V. et al. Risk factors associated with early-onset colorectal cancer. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2019.10.009 (2019).
Low, E. E. et al. Risk factors for early-onset colorectal cancer. Gastroenterology 159, 492–501.e7 (2020).
Nguyen, L. H. et al. Sedentary behaviors, TV viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr. 2, pky073 (2018).
Liu, P. H. et al. Association of obesity with risk of early-onset colorectal cancer among women. JAMA Oncol. 5, 37–44 (2019).
Liang, P. S., Mayer, J. D., Wakefield, J. & Ko, C. W. Temporal trends in geographic and sociodemographic disparities in colorectal cancer among medicare patients, 1973–2010. J. Rural Health 33, 361–370 (2017).
Murphy, C. C., Wallace, K., Sandler, R. S. & Baron, J. A. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology 156, 958–965 (2019).
Mayer-Davis, E. J. et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N. Engl. J. Med. 376, 1419–1429 (2017).
Ogden, C. L. et al. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA 315, 2292–2299 (2016).
Holowatyj, A. N., Ruterbusch, J. J., Rozek, L. S., Cote, M. L. & Stoffel, E. M. Racial/Ethnic disparities in survival among patients with young-onset colorectal cancer. J. Clin. Oncol. 34, 2148–2156 (2016).
Archambault, A. N. et al. Cumulative burden of colorectal cancer-associated genetic variants is more strongly associated with early-onset vs late-onset cancer. Gastroenterology 158, 1274–1286.e12 (2020).
Wang, L. et al. Risk factor profiles differ for cancers of different regions of the colorectum. Gastroenterology 159, 241–256.e13 (2020).
Kneuertz, P. J. et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 150, 402–409 (2015).
Myers, E. A. et al. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J. Gastroenterol. 19, 5651–5657 (2013).
Chen, F. W., Sundaram, V., Chew, T. A. & Ladabaum, U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin. Gastroenterol. Hepatol. 15, 728–737.e3 (2017).
Deng, S. X. et al. Factors influencing diagnosis of colorectal cancer: a hospital-based survey in China. J. Dig. Dis. 13, 517–524 (2012).
Ben-Ishay, O. et al. Diagnosis of colon cancer differs in younger versus older patients despite similar complaints. Isr. Med. Assoc. J. 15, 284–287 (2013).
Liang, J. T. et al. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br. J. Surg. 90, 205–214 (2003).
Siegel, R. L., Jakubowski, C. D., Fedewa, S. A., Davis, A. & Azad, N. S. Colorectal cancer in the young: epidemiology, prevention, management. Am. Soc. Clin. Oncol. Educ. Book 40, 1–14 (2020).
Stoffel, E. M. et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology 154, 897–905.e1 (2018).
Pearlman, R. et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. 3, 464–471 (2017).
Mork, M. E. et al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J. Clin. Oncol. 33, 3544–3549 (2015).
Valle, L., Vilar, E., Tavtigian, S. V. & Stoffel, E. M. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J. Pathol. 247, 574–588 (2019).
Koskenvuo, L., Ryynänen, H. & Lepistö, A. Timing of prophylactic colectomy in familial adenomatous polyposis. Colorectal Dis. https://doi.org/10.1111/codi.15151 (2020).
Ogino, S. et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut 67, 1168–1180 (2018).
Yamauchi, M. et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 61, 847–854 (2012).
Yamauchi, M. et al. Colorectal cancer: a tale of two sides or a continuum? Gut 61, 794–797 (2012).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin. Transl Gastroenterol. 7, e200 (2016).
Holowatyj, A. N. et al. Clinicopathologic and racial/ethnic differences of colorectal cancer among adolescents and young adults. Clin. Transl Gastroenterol. 10, e00059 (2019).
Willauer, A. N. et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer 125, 2002–2010 (2019).
Perea, J. et al. Age at onset should be a major criterion for subclassification of colorectal cancer. J. Mol. Diagn. 16, 116–126 (2014).
Berg, M. et al. Distinct high resolution genome profiles of early onset and late onset colorectal cancer integrated with gene expression data identify candidate susceptibility loci. Mol. Cancer 9, 100 (2010).
Arriba, M. et al. DNA copy number profiling reveals different patterns of chromosomal instability within colorectal cancer according to the age of onset. Mol. Carcinog. 55, 705–716 (2016).
Yeo, H. et al. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin. Colorectal Cancer 16, 293–299.e6 (2017).
Soliman, B. G., Karagkounis, G., Church, J. M., Plesec, T. & Kalady, M. F. Mucinous histology signifies poor oncologic outcome in young patients with colorectal cancer. Dis. Colon Rectum 61, 547–553 (2018).
Rodriguez, L. et al. Disease characteristics, clinical management, and outcomes of young patients with colon cancer: a population-based study. Clin. Colorectal Cancer 17, e651–e661 (2018).
Nagtegaal, I., Arends, M. & Salto-Tellez, M. in WHO Classification of Tumours, 5th edition: Digestive System Tumours (ed. WHO Classification of Tumours Editorial Board) Vol. 180 (World Health Organization, 2019).
Inamura, K. et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann. Surg. Oncol. 22, 1226–1235 (2015).
Lieu, C. H. et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin. Cancer Res. 25, 5852–5858 (2019).
Antelo, M. et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS ONE 7, e45357 (2012).
Feinberg, A. P. The key role of epigenetics in human disease prevention and mitigation. N. Engl. J. Med. 378, 1323–1334 (2018).
Baba, Y. et al. Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol. Cancer 9, 125 (2010).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
Lieu, C. H. et al. Association of age with survival in patients with metastatic colorectal cancer: analysis from the ARCAD Clinical Trials Program. J. Clin. Oncol. 32, 2975–2984 (2014).
Sultan, I. et al. Distinct features of colorectal cancer in children and adolescents: a population-based study of 159 cases. Cancer 116, 758–765 (2010).
Kim, T. J., Kim, E. R., Hong, S. N., Chang, D. K. & Kim, Y. H. Long-term outcome and prognostic factors of sporadic colorectal cancer in young patients: a large institutional-based retrospective study. Medicine 95, e3641 (2016).
Chou, C. L., Tseng, C. J. & Shiue, Y. L. The impact of young age on the prognosis for colorectal cancer: a population-based study in Taiwan. Jpn. J. Clin. Oncol. 47, 1010–1018 (2017).
Blanke, C. D. et al. Impact of young age on treatment efficacy and safety in advanced colorectal cancer: a pooled analysis of patients from nine first-line phase III chemotherapy trials. J. Clin. Oncol. 29, 2781–2786 (2011).
Pokharkar, A. B. et al. Young vs old colorectal cancer in indian subcontinent: a tertiary care center experience. Indian J. Surg. Oncol. 8, 491–498 (2017).
Rho, Y. S. et al. Comparing clinical characteristics and outcomes of young-onset and late-onset colorectal cancer: an international collaborative study. Clin. Colorectal Cancer 16, 334–342 (2017).
Yang, Z. et al. Characteristics and long-term survival of colorectal cancer patients aged 44 years and younger. Clin. Transl Oncol. 14, 896–904 (2012).
McMillan, D. C. & McArdle, C. S. The impact of young age on cancer-specific and non-cancer-related survival after surgery for colorectal cancer: 10-year follow-up. Br. J. Cancer 101, 557–560 (2009).
Manjelievskaia, J. et al. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 152, 452–459 (2017).
Schellerer, V. S. et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int. J. Colorectal Dis. 27, 71–79 (2012).
Wang, M. J. et al. The prognostic factors and multiple biomarkers in young patients with colorectal cancer. Sci. Rep. 5, 10645 (2015).
Quah, H. M. et al. Young age influences treatment but not outcome of colon cancer. Ann. Surg. Oncol. 14, 2759–2765 (2007).
Boyce, S. et al. Young-onset colorectal cancer in New South Wales: a population-based study. Med. J. Aust. 205, 465–470 (2016).
O’Connell, J. B. et al. Do young colon cancer patients have worse outcomes? World J. Surg. 28, 558–562 (2004).
Hubbard, J. et al. Benefits and adverse events in younger versus older patients receiving adjuvant chemotherapy for colon cancer: findings from the Adjuvant Colon Cancer Endpoints data set. J. Clin. Oncol. 30, 2334–2339 (2012).
Kolarich, A. et al. Rectal cancer patients younger than 50 years lack a survival benefit from NCCN guideline-directed treatment for stage II and III disease. Cancer 124, 3510–3519 (2018).
Abdelsattar, Z. M. et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 122, 929–934 (2016).
Orsini, R. G. et al. Comparable survival for young rectal cancer patients, despite unfavourable morphology and more advanced-stage disease. Eur. J. Cancer 51, 1675–1682 (2015).
Hofseth, L. J. et al. Early-onset colorectal cancer: initial clues and current views. Nat. Rev. Gastroenterol. Hepatol. 17, 352–364 (2020).
Ruder, E. H. et al. Adolescent and mid-life diet: risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 94, 1607–1619 (2011).
van der Pols, J. C. et al. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am. J. Clin. Nutr. 86, 1722–1729 (2007).
Bjørge, T., Engeland, A., Tverdal, A. & Smith, G. D. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am. J. Epidemiol. 168, 30–37 (2008).
Hughes, L. A. et al. Early life exposure to famine and colorectal cancer risk: a role for epigenetic mechanisms. PLoS ONE 4, e7951 (2009).
Hughes, L. A. et al. Childhood and adolescent energy restriction and subsequent colorectal cancer risk: results from the Netherlands Cohort Study. Int. J. Epidemiol. 39, 1333–1344 (2010).
Boice J. D. Jr in Cancer Epidemiology and Prevention (eds Schottenfeld, D. & Fraumeni, J. F. Jr) 259–293 (Oxford University Press, 2006).
Sigurdson, A. J. et al. Primary thyroid cancer after a first tumour in childhood (the childhood cancer survivor study): a nested case-control study. Lancet 365, 2014–2023 (2005).
Caprio, S. et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am. J. Physiol. 269, E118–E126 (1995).
Chiarelli, F. & Marcovecchio, M. L. Insulin resistance and obesity in childhood. Eur. J. Endocrinol. 159 (Suppl. 1), S67–S74 (2008).
Loeb, L. A. Human cancers express a mutator phenotype: hypothesis, origin, and consequences. Cancer Res. 76, 2057–2059 (2016).
Tomasetti, C. & Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347, 78–81 (2015).
Popkin, B. M., Adair, L. S. & Ng, S. W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70, 3–21 (2012).
Stephen, A. M. & Wald, N. J. Trends in individual consumption of dietary fat in the United States, 1920–1984. Am. J. Clin. Nutr. 52, 457–469 (1990).
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390, 2627–2642 (2017).
Centers for Disease Control and Prevention (CDC). Achievements in public health, 1900–1999: healthier mothers and babies. Morbidity Mortal. Wkly. Rep. 48, 849–858 (1999).
Simmons, H. E. & Stolley, P. D. This is medical progress? Trends and consequences of antibiotic use in the United States. JAMA 227, 1023–1028 (1974).
O’Sullivan, A., Farver, M. & Smilowitz, J. T. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights 8 (Suppl. 1), 1–9 (2015).
Lu, Y. H., Wang, N. & Jin, F. Long-term follow-up of children conceived through assisted reproductive technology. J. Zhejiang Univ. Sci. B 14, 359–371 (2013).
Sandall, J. et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 392, 1349–1357 (2018).
Zhou, Q. et al. Risk of colorectal cancer in ulcerative colitis patients: a systematic review and meta-analysis. Gastroenterol. Res. Pract. 2019, 5363261 (2019).
Ghione, S. et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease (1988–2011): a population-based study of french adolescents. Am. J. Gastroenterol. 113, 265–272 (2018).
Shendure, J. & Akey, J. M. The origins, determinants, and consequences of human mutations. Science 349, 1478–1483 (2015).
Strum, W. B. & Boland, C. R. Clinical and genetic characteristics of colorectal cancer in persons under 50 years of age: a review. Dig. Dis. Sci. 64, 3059–3065 (2019).
Poon, S. L., McPherson, J. R., Tan, P., Teh, B. T. & Rozen, S. G. Mutation signatures of carcinogen exposure: genome-wide detection and new opportunities for cancer prevention. Genome Med. 6, 24 (2014).
Ogino, S., Nowak, J. A., Hamada, T., Milner, D. A. Jr & Nishihara, R. Insights into pathogenic interactions among environment, host, and tumor at the crossroads of molecular pathology and epidemiology. Annu. Rev. Pathol. 14, 83–103 (2019).
Hur, K. et al. Hypomethylation of long interspersed nuclear element-1 (LINE-1) leads to activation of proto-oncogenes in human colorectal cancer metastasis. Gut 63, 635–646 (2014).
Cho, Y. H. et al. LINE-1 hypomethylation is associated with radiation-induced genomic instability in industrial radiographers. Env. Mol. Mutagen. 60, 174–184 (2019).
Gogna, P., O’Sullivan, D. E. & King, W. D. The effect of inflammation-related lifestyle exposures and interactions with gene variants on long interspersed nuclear element-1 DNA methylation. Epigenomics 10, 785–796 (2018).
Martin, E. M. & Fry, R. C. Environmental influences on the epigenome: exposure- associated DNA methylation in human populations. Annu. Rev. Public Health 39, 309–333 (2018).
Yan, H. H. N. et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut https://doi.org/10.1136/gutjnl-2019-320019 (2020).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019).
Chen, B., Du, G., Guo, J. & Zhang, Y. Bugs, drugs, and cancer: can the microbiome be a potential therapeutic target for cancer management? Drug. Discov. Today 24, 1000–1009 (2019).
Mima, K. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 65, 1973–1980 (2016).
Brennan, C. A. & Garrett, W. S. Fusobacterium nucleatum - symbiont, opportunist and oncobacterium. Nat. Rev. Microbiol. 17, 156–166 (2019).
Pleguezuelos-Manzano, C. et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273 (2020).
Dejea, C. M. et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl Acad. Sci. USA 111, 18321–18326 (2014).
Flemer, B. et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 66, 633–643 (2017).
Phipps, A. I., Chan, A. T. & Ogino, S. Anatomic subsite of primary colorectal cancer and subsequent risk and distribution of second cancers. Cancer 119, 3140–3147 (2013).
Mima, K. et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 1, 653–661 (2015).
Kosumi, K. et al. The amount of bifidobacterium genus in colorectal carcinoma tissue in relation to tumor characteristics and clinical outcome. Am. J. Pathol. 188, 2839–2852 (2018).
Kim, J. Y. et al. Different risk factors for advanced colorectal neoplasm in young adults. World J. Gastroenterol. 22, 3611–3620 (2016).
Blaser, M. J. & Dominguez-Bello, M. G. The human microbiome before birth. Cell Host Microbe 20, 558–560 (2016).
Esaiassen, E., Fjalstad, J. W., Juvet, L. K., van den Anker, J. N. & Klingenberg, C. Antibiotic exposure in neonates and early adverse outcomes: a systematic review and meta-analysis. J. Antimicrob. Chemother. 72, 1858–1870 (2017).
Azad, M. B. et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993 (2016).
Klein, E. Y. et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl Acad. Sci. USA 115, E3463–E3470 (2018).
Park, J. E., Jardine, L., Gottgens, B., Teichmann, S. A. & Haniffa, M. Prenatal development of human immunity. Science 368, 600–603 (2020).
Cao, Y. et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut 67, 672–678 (2018).
Zhang, J. et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989–2012: a matched case-control study. Gut 68, 1971–1978 (2019).
Kilkkinen, A. et al. Antibiotic use predicts an increased risk of cancer. Int. J. Cancer 123, 2152–2155 (2008).
Mauri, G. et al. Early-onset colorectal cancer in young individuals. Mol. Oncol. 13, 109–131 (2019).
Ganal-Vonarburg, S. C., Hornef, M. W. & Macpherson, A. J. Microbial-host molecular exchange and its functional consequences in early mammalian life. Science 368, 604–607 (2020).
Abualkhair, W. H. et al. Trends in incidence of early-onset colorectal cancer in the United States among those approaching screening age. JAMA Netw. Open 3, e1920407 (2020).
Ladabaum, U., Dominitz, J. A., Kahi, C. & Schoen, R. E. Strategies for colorectal cancer screening. Gastroenterology 158, 418–432 (2020).
Enwerem, N. et al. Systematic review of prevalence, risk factors, and risk for metachronous advanced neoplasia in patients with young-onset colorectal adenoma. Clin. Gastroenterol. Hepatol. https://doi.org/10.1016/j.cgh.2020.04.092 (2020).
Rex, D. K. et al. Colorectal cancer screening: recommendations for physicians and patients from the US multi-society task force on colorectal cancer. Gastroenterology 153, 307–323 (2017).
Levin, B. et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 134, 1570–1595 (2008).
Giardiello, F. M. et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US multi-society task force on colorectal cancer. Am. J. Gastroenterol. 109, 1159–1179 (2014).
Wolf, A. M. D. et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 68, 250–281 (2018).
Fedewa, S. A., Siegel, R. L., Goding Sauer, A., Bandi, P. & Jemal, A. Colorectal cancer screening patterns after the American Cancer Society’s recommendation to initiate screening at age 45 years. Cancer 126, 1351–1353 (2020).
U. S. Preventive Services Task Force. et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 315, 2564–2575 (2016).
Peterse, E. F. P. et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 124, 2964–2973 (2018).
Ogino, S., Chan, A. T., Fuchs, C. S. & Giovannucci, E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 60, 397–411 (2011).
Hughes, L. A. E., Simons, C., van den Brandt, P. A., van Engeland, M. & Weijenberg, M. P. Lifestyle, diet, and colorectal cancer risk according to (Epi)genetic instability: current evidence and future directions of molecular pathological epidemiology. Curr. Colorectal Cancer Rep. 13, 455–469 (2017).
Gunter, M. J. et al. Meeting report from the joint IARC-NCI international cancer seminar series: a focus on colorectal cancer. Ann. Oncol. 30, 510–519 (2019).
Carr, P. R. et al. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann. Oncol. 29, 825–834 (2018).
Inamura, K. Roles of microbiota in response to cancer immunotherapy. Semin. Cancer Biol. 65, 164–175 (2020).
Rescigno, T., Micolucci, L., Tecce, M. F. & Capasso, A. Bioactive nutrients and nutrigenomics in age-related diseases. Molecules 22, 105 (2017).
Wang, S. T. et al. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 26, 562–597 (2020).
Amitay, E. L. et al. Association of aspirin and nonsteroidal anti-inflammatory drugs with colorectal cancer risk by molecular subtypes. J. Natl Cancer Inst. 111, 475–483 (2019).
Luo, K. et al. Fusobacterium nucleatum, the communication with colorectal cancer. Biomed. Pharmacother. 116, 108988 (2019).
Murphy, N., Jenab, M. & Gunter, M. J. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat. Rev. Gastroenterol. Hepatol. 15, 659–670 (2018).
Rajpoot, M., Sharma, A. K., Sharma, A. & Gupta, G. K. Understanding the microbiome: emerging biomarkers for exploiting the microbiota for personalized medicine against cancer. Semin. Cancer Biol. 52, 1–8 (2018).
Chavarro, J. E. et al. Contributions of the nurses’ health studies to reproductive health research. Am. J. Public Health 106, 1669–1676 (2016).
Gupta, S. et al. Potential impact of family history-based screening guidelines on the detection of early-onset colorectal cancer. Cancer 126, 3013–3020 (2020).
Cirillo, P. M. & Cohn, B. A. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the child health and development studies pregnancy cohort. Circulation 132, 1234–1242 (2015).
Dwyer, A. J. et al. A summary of the fight colorectal cancer working meeting: exploring risk factors and etiology of sporadic early-age onset colorectal cancer. Gastroenterology 157, 280–288 (2019).
Morton, S. M. et al. Cohort profile: growing up in New Zealand. Int. J. Epidemiol. 42, 65–75 (2013).
Connelly, R. & Platt, L. Cohort profile: UK millennium cohort study (MCS). Int. J. Epidemiol. 43, 1719–1725 (2014).
Nishi, A. et al. Lifecourse epidemiology and molecular pathological epidemiology. Am. J. Prev. Med. 48, 116–119 (2015).
Kim, N. H. et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20–39 years old). Clin. Gastroenterol. Hepatol. 17, 115–122 (2019).
Acknowledgements
The authors thank Tyler Twombly (Program in Molecular Pathological Epidemiology, Department of Pathology, Brigham and Women’s Hospital, Boston, USA) for proofreading of the manuscript. The work of the authors is supported by US NIH grants (R35 CA197735 and R01 CA248857 to S.O., R21 CA230873 to S.O. and K.W., R01 CA205406 to K.N., R03 CA197879 to K.W., and R37 CA246175 to Y.C.), a Cancer Research UK Grand Challenge Award (UK C10674/A27140 to M.G., K.N. and S.O.), an Investigator Initiated Grant from the American Institute for Cancer Research (AICR) to K.W., and by a US Department of Defense grant (CA160344 to K.N.). K.N. and M.G. have received support from the Project P Fund. The work of M.G. has been supported by a Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17) administered by the American Association for Cancer Research, a scientific partner of SU2C. The work of T.U. has been supported by an Overseas Research Fellowship grant (201960541) from the Japan Society for the Promotion of Science, the Uehara Memorial Foundation and the Yasuda Medical Foundation. The work of K.F. has been supported by fellowship grants from the Uehara Memorial Foundation and the Grant of The Clinical Research Promotion Foundation (2018). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the conceptualization of the article and approved the final version of the manuscript. S.O. developed the initial basic concept and supervised the manuscript preparation. N.A., T.U., R.Z., K.F. and S.O. constructed the overall outline of the manuscript, searched the literature and drafted the manuscript. T.H., M.G., K.W., Y.C., K.N. and S.O. revised the manuscript critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
K.N. has received institutional research funding from Evergrande Group, Genentech, Gilead Sciences, Pharmavite, Revolution Medicines, Tarrex Biopharma and Trovagene, and has served on advisory boards for Array Biopharma, Bayer and Seattle Genetics. M.G. has received research funding from Bristol-Myers Squibb and Merck. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks Samir Gupta, Jose Perea and Benjamin Weinberg for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Child Health and Development Studies (CHDS): http://www.chdstudies.org/
Colon Cancer Family Registry (CCFR) Cohort: https://www.coloncfr.org/
Growing Up in New Zealand: https://www.growingup.co.nz/
Growing Up Today Study 2 (GUTS2): https://nhs2survey.org/gutswordpress/index.php/about/history/
Millennium Cohort Study: https://cls.ucl.ac.uk/cls-studies/millennium-cohort-study/
National Children’s Study: https://www.nichd.nih.gov/research/supported/NCS
Nurses’ Health Study 3 (NHS3): https://www.nhs3.org/
ORIGINS Project: https://originsproject.telethonkids.org.au/
SEER Cancer Statistics Review 1975–2017: https://seer.cancer.gov/csr/1975_2017/
Rights and permissions
About this article
Cite this article
Akimoto, N., Ugai, T., Zhong, R. et al. Rising incidence of early-onset colorectal cancer — a call to action. Nat Rev Clin Oncol 18, 230–243 (2021). https://doi.org/10.1038/s41571-020-00445-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-020-00445-1