Abstract
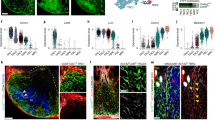
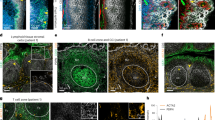
Fibroblastic reticular cells (FRCs) and their specialized collagen fibers termed ‘conduits’ form fundamental structural units supporting lymphoid tissues. In lymph nodes, conduits are known to transport interstitial fluid and small molecules from afferent lymphatics into the nodal parenchyma. However, the immunological contributions of conduit function have remained elusive. Here, we report that intestinal Peyer’s patches (PPs) contain a specialized conduit system that directs the flow of water absorbed across the intestinal epithelium. Notably, PP FRCs responded to conduit fluid flow via the mechanosensitive ion channel Piezo1. Disruption of fluid flow or genetic deficiency of Piezo1 on CCL19-expressing stroma led to profound structural alterations in perivascular FRCs and associated high endothelial venules. This in turn impaired lymphocyte entry into PPs and initiation of mucosal antibody responses. These results identify a critical role for conduit-mediated fluid flow in the maintenance of PP homeostasis and mucosal immunity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Microarrays are available on the Gene Expression Omnibus database with the accession number GSE135612.
References
Anderson, A. O. & Anderson, N. D. Studies on the structure and permeability of the microvasculature in normal rat lymph nodes. Am. J. Pathol. 80, 387–418 (1975).
Gretz, J. E., Anderson, A. O. & Shaw, S. Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol. Rev. 156, 11–24 (1997).
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Cremasco, V. et al. B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat. Immunol. 15, 973–981 (2014).
Gunn, M. D. et al. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature 391, 799–803 (1998).
Hase, H. et al. BAFF/BLyS can potentiate B-cell selection with the B-cell coreceptor complex. Blood 103, 2257–2265 (2004).
Link, A. et al. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 8, 1255–1265 (2007).
Luther, S. A., Tang, H. L., Hyman, P. L., Farr, A. G. & Cyster, J. G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl Acad. Sci. USA 97, 12694–12699 (2000).
Roozendaal, R. et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity 30, 264–276 (2009).
Sainte-Marie, G. & Peng, F. S. Diffusion of a lymph-carried antigen in the fiber network of the lymph node of the rat. Cell Tissue Res. 245, 481–486 (1986).
Sixt, M. et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005).
Baekkevold, E. S. et al. The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J. Exp. Med. 193, 1105–1112 (2001).
Gretz, J. E., Norbury, C. C., Anderson, A. O., Proudfoot, A. E. & Shaw, S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J. Exp. Med. 192, 1425–1440 (2000).
Stein, J. V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Hendriks, H. R., Duijvestijn, A. M. & Kraal, G. Rapid decrease in lymphocyte adherence to high endothelial venules in lymph nodes deprived of afferent lymphatic vessels. Eur. J. Immunol. 17, 1691–1695 (1987).
Hendriks, H. R. & Eestermans, I. L. Disappearance and reappearance of high endothelial venules and immigrating lymphocytes in lymph nodes deprived of afferent lymphatic vessels: a possible regulatory role of macrophages in lymphocyte migration. Eur. J. Immunol. 13, 663–669 (1983).
Mebius, R. E. et al. Expression of GlyCAM-1, an endothelial ligand for L-selectin, is affected by afferent lymphatic flow. J. Immunol. 151, 6769–6776 (1993).
Berg, E. L., McEvoy, L. M., Berlin, C., Bargatze, R. F. & Butcher, E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 366, 695–698 (1993).
Thierry, G. R. et al. The conduit system exports locally secreted IgM from lymph nodes. J. Exp. Med. 215, 2972–2983 (2018).
Rantakari, P. et al. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat. Immunol. 16, 386–396 (2015).
Gonzalez, S. F. et al. Trafficking of B cell antigen in lymph nodes. Annu. Rev. Immunol. 29, 215–233 (2011).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Ranade, S. S. et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl Acad. Sci. USA 111, 10347–10352 (2014).
Rodda, L. B., Bannard, O., Ludewig, B., Nagasawa, T. & Cyster, J. G. Phenotypic and morphological properties of germinal center dark zone Cxcl12-expressing reticular cells. J. Immunol. 195, 4781–4791 (2015).
Rodda, L. B. et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 48, 1014–1028.e6 (2018).
Anderson, A. O. & Shaw, S. T cell adhesion to endothelium: the FRC conduit system and other anatomic and molecular features which facilitate the adhesion cascade in lymph node. Semin. Immunol. 5, 271–282 (1993).
Gretz, J. E., Kaldjian, E. P., Anderson, A. O. & Shaw, S. Sophisticated strategies for information encounter in the lymph node: the reticular network as a conduit of soluble information and a highway for cell traffic. J. Immunol. 157, 495–499 (1996).
Moe, R. E. Fine structure of the reticulum and sinuses of lymph nodes. Am. J. Anat. 110, 217–257 (1963).
Tomei, A. A., Siegert, S., Britschgi, M. R., Luther, S. A. & Swartz, M. A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183, 4273–4283 (2009).
Abbott, R. K. et al. Precursor frequency and affinity determine B cell competitive fitness in germinal centers, tested with germline-targeting HIV vaccine immunogens. Immunity 48, 133–146.e6 (2018).
Jenkins, M. K. & Moon, J. J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 188, 4135–4140 (2012).
Moon, J. J. et al. Naive CD4+ T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 (2007).
Obar, J. J., Khanna, K. M. & Lefrancois, L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity 28, 859–869 (2008).
Cahalan, S. M. et al. Piezo1 links mechanical forces to red blood cell volume. eLife 4, e07370 (2015).
Degn, S. E., Alicot, E. & Carroll, M. C. B cell tolerance to epidermal ribonuclear-associated neo-autoantigen in vivo. Clin. Exp. Immunol. 191, 151–165 (2018).
Chai, Q. et al. Maturation of lymph node fibroblastic reticular cells from myofibroblastic precursors is critical for antiviral immunity. Immunity 38, 1013–1024 (2013).
Fletcher, A. L. et al. Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front. Immunol. 2, 35 (2011).
Islam, S. et al. Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods 11, 163–166 (2014).
Huber, W. et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat. Methods 12, 115–121 (2015).
Wu, T. D. & Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26, 873–881 (2010).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 15, R29 (2014).
Acknowledgements
We thank the members of the Carroll laboratory and the Turley laboratory for comments and suggestions on the study. We thank B. Ludewig (Kantonsspital St. Gallen) for providing Ccl19Cre mice. This study was funded in part by Genentech and by the US National Institutes of Health (grant nos. F31 DK1055 to J.E.C.; R33 AI110164 and RO1 AI130307 to M.C.C.; RO1 DK074500 and R21 CA182598 to S.J.T.). We thank S. Jhunjhunwala and N. Lounsbury for management of sequencing files and data processing.
Author information
Authors and Affiliations
Contributions
J.E.C. designed and performed experiments, analyzed results and wrote the manuscript. M.B.B. performed experiments, analyzed results and contributed to the writing of the manuscript. E.G. performed experiments and analyzed results. M.C.C. and S.J.T. designed and supervised the study and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.J.T. and M.B.B are employed by Genentech.
Additional information
Peer review information Laurie A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
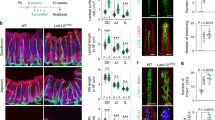
Supplementary Figure 1 Stromal cell numbers in PPs following treatments with PEG or amiloride.
(a,b) Flow cytometric analysis PP stromal cell populations (CD45−TER119−) following treatment with PEG or amiloride. Subpopulations are defined as FRC (PDPN+CD31−), LEC (PDPN+CD31+), BEC (PDPN−CD31+, and HEV (CD31+MAdCAM+). Mice were treated for a period of 3 days (a; n = 6 mice per group) or one week (b; n = 15 (Ctl, PEG) or 5 (Aml) mice per group). Representative FACS plots illustrate relative composition of FRCs (gp38+CD31−), LECs (gp38+CD31+), and BECs (gp38−CD31+). Data are graphed as relative cell counts. For all graphs, data represented as mean ± SEM. Groups were compared by one way ANOVA. *P < 0.05, **P < 0.01, ****P < 0.0001. No statistical significance is indicated with ‘ns’.
Supplementary Figure 2 Blockade of fluid absorption equally affects B and T cell migration and total cellularity in the PP, but not myeloid cell populations.
(a) Frequency of total B and T lymphocytes resident in the PP, MLN, ILN, and spleen of control, PEG-treated, or amiloride-treated mice. (n = 9 (Ctl) or 12 (PEG, Aml) mice per group). (b) Flow cytometric analysis of myeloid cell populations from the PP of control or PEG-treated mice (n=8 (Ctl, PEG) or 4 (Aml) mice per group) (c) Flow cytometric analysis of mice following a 3-day treatment period with PEG or amiloride. Mice were additionally treated with either FTY720 or PBS on day 0 and day 2. Lymphocyte cellularity was analyzed from PP (n= 9 (Ctl, PEG) or 5 (Aml) mice per group) and ILN (n=5 mice per group). Data represented as mean ± SEM. Groups were compared by one way ANOVA. * (P ≤ 0.05), ** (P ≤ 0.01), *** (P ≤ 0.005). No statistical significance is indicated with ‘ns’.
Supplementary Figure 3 Altered LN and PP cellularity is not a consequence of dehydration.
(a) Flow cytometric analysis of total lymphocyte counts from PP and LN of mice after the following treatments: control untreated, PEG for 3 days, PEG for 3 days then restored to normal drinking water for 12 hours (PEG > Water). (n=11 (Ctl, PEG) or 12 (PEG > Water) mice per group). (b) Flow cytometric analysis of total lymphocyte counts from PP and LN after the following treatments: control untreated, PEG for 3 days, amiloride for 3 days, PEG or amiloride for 3 days with twice daily subcutaneous injection of saline. (n = 13 (Ctl, PEG) or 8 (Aml) mice per group). Data represented as mean ± SEM. Groups were compared by one way ANOVA. * (P ≤ 0.05), ** (P ≤ 0.01), *** (P ≤ 0.005). No statistical significance is indicated with ‘ns’.
Supplementary Figure 4 Conditional ablation of Piezo1 in FRCs.
(a) Transcriptional expression measured by RNAseq of Piezo1 in FRCs and HEV ECs isolated from the PP of control or PEG-treated mice. (n= 7 mice per group (FRC) or 4 mice per group (HEV EC)) (b) Validation of specific gene recombination in exons 20-23 of Piezo1. Genomic DNA isolated from PP stromal cells enriched by depletion of CD45+ cells using magnetic beads, primary FRCs expanded in culture for 5 days, or tail snips. Genotypes of mice indicated below each lane. Bands indicate the following: Intact gene flanked by flox sequences = 330 bp, knockout = 230bp, wildtype = 160bp. (c) Flow cytometric analysis of the distribution of eYFP-expressing cells isolated from the PP and inguinal LNs of Ccl19cre+ x ROSA26-eYFP mice. (n=3 mice per group). (d) Confocal microscopy of cultured PP FRCs. FRCs were enriched and culture expanded from Ccl19cre+ x ROSA26-eYFP mice. Representative of two independent experiments. (e) Transcriptional expression of Piezo1 in PP FRCs isolated from WT and Ccl19-P1cKO mice. FRCs were enriched and culture expanded before mRNA was measured by qPCR (n= 4 (Cre−) or 3 (Cre+) mice). Data represented as mean ± SEM. Groups were compared by unpaired two tailed Student’s t test. * (P ≤ 0.05), ** (P ≤ 0.01), *** (P ≤ 0.005). No statistical significance is indicated with ‘ns’.
Supplementary Figure 5 Specific expression of Ccl19cre transgene in the FRC population.
(a,b) Confocal imaging analysis of the distribution of eYFP-expressing cells in the intestinal PP of Ccl19cre+ x ROSA26-eYFP mice. Representative of three experiments (a) FDCs are identified by positive staining with anti-CR1/CR2 (blue); MRCs are identified by positive staining with anti-RANKL (Red); FRCs are identified by the positive staining with anti-PDPN (grey) and absence of staining with anti-CR1/CR2 and anti-RANKL. Left image scale bar = 100um. Right images scale bar = 20um. (b) Representative confocal image of an HEV in cross-section. HEV endothelial cells are identified by positive staining with anti-MAdCAM (red). Scale bar = 10um.
Supplementary Figure 6 Altered LN and PP cellularity in Ccl19-P1cKO mice.
(a) Flow cytometric analysis of total stromal cell composition from the LN and PPs of Ccl19-P1cKO and Ccl19Cre − x Piezo1fl/fl (WT) mice. (n=3 mice per group). (b) Confocal imaging of lymphatic vessel architecture (identified by positive staining with anti-LYVE1) within the PP of control, PEG-treated or Ccl19-P1cKO mice. Representative of two independent experiments. (c) Flow cytometric analysis of B cell and T cell numbers from PP, MLN, and ILN of Ccl19-P1cKO and Ccl19Cre − x Piezo1fl/fl (WT) mice. Presented as relative cell counts (n=14 mice per group (PP, ILN) or 7 mice per group (MLN)). (d) Transcriptional expression measured by qPCR of Piezo1 in FRCs isolated from the LN and PP of untreated WT mice. (n=6 mice per group). For all graphs, data represented as mean ± SEM. Groups were compared by two way ANOVA (c) or unpaired two tailed Student’s t test (d). *P < 0.05, **P < 0.01, ****P < 0.0001. No statistical significance is indicated with ‘ns’.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6.
Supplementary Video 1
Lymphocyte extravasation in PP of PEG-treated mouse. Mice were treated for 3 d with PEG in drinking water before imaging by multiphoton intravital microscopy. Labeled splenocytes (green) were adoptively transferred along with a Qtracker 655 vascular label (red) for visualization of the vasculature. Imaging began within 30–60 min of lymphocyte transfer. Time is shown in minutes and seconds. Video is representative of three independent experiments.
Supplementary Video 2
Lymphocyte extravasation in PP of amiloride-treated mouse. Mice were treated with amiloride 3 d before imaging by multiphoton intravital microscopy. Labeled splenocytes (green) were adoptively transferred along with a Qtracker 655 vascular label (red) for visualization of the vasculature. Imaging began within 30–60 min of lymphocyte transfer. Time is shown in minutes and seconds. Video is representative of two independent experiments.
Supplementary Video 3
Lymphocyte extravasation in PP of untreated mouse. Untreated mice were imaged by multiphoton intravital microscopy. Labeled splenocytes (green) were adoptively transferred along with a Qtracker 655 vascular label (red) for visualization of the vasculature. Imaging began within 30–60 min of lymphocyte transfer. Time is shown in minutes and seconds. Video is representative of three independent experiments.
Supplementary Video 4
Lymphocyte extravasation in PP of anti-MAdCAM1-treated mouse. Mice were treated with blocking anti-MAdCAM1 immediately before imaging by multiphoton intravital microscopy. Labeled splenocytes (green) were adoptively transferred along with a Qtracker 655 vascular label (red) for visualization of the vasculature. Imaging began within 30–60 min of lymphocyte transfer. Time is shown in minutes and seconds. Video is representative of two independent experiments.
Rights and permissions
About this article
Cite this article
Chang, J.E., Buechler, M.B., Gressier, E. et al. Mechanosensing by Peyer’s patch stroma regulates lymphocyte migration and mucosal antibody responses. Nat Immunol 20, 1506–1516 (2019). https://doi.org/10.1038/s41590-019-0505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0505-z
This article is cited by
-
Tango of B cells with T cells in the making of secretory antibodies to gut bacteria
Nature Reviews Gastroenterology & Hepatology (2023)
-
TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection
Nature Communications (2021)
-
Fibroblastic reticular cell lineage convergence in Peyer’s patches governs intestinal immunity
Nature Immunology (2021)
-
Cells of the human intestinal tract mapped across space and time
Nature (2021)
-
The mesenchymal context in inflammation, immunity and cancer
Nature Immunology (2020)