Genetics
Funky Valentine: Love at First Whiff?
Partner choice, attractive scents and the immune system
Posted February 14, 2019

Charles Darwin’s fascination with mating signals between males and females led him to recognize sexual selection: Preferences of one sex impose selection pressure on the other, driving evolution of special, sometimes spectacular features. Peacocks’ tails and the red bellies of breeding male sticklebacks are well-known examples.

Some primates also show remarkable features attributed to sexual selection. The adult male mandrill’s blue-and-red face is perhaps the most impressive. Males also differ strikingly from females in other prominent characteristics such as overall body size and enlarged, dagger-like canine teeth. They are said to have twin functions, equipping males to fight for access to females, but simultaneously serving as signals that attract females.

Primates like mandrills live in large groups containing several promiscuously mating adult males and females. Marked dominance relationships among males, governing access to both females and resources, are common. It is often assumed that the highest-ranking (alpha) male necessarily sires most offspring in his group because his “good genes” benefit all females that mate with him.
An alternative perspective
In a previous blog post about mate choice, I questioned the notion that all females in a group benefit from having offspring sired by the dominant male. This male-oriented “one-size-fits-all” notion treats females as passive responders to powerfully built macho males with prominent canine teeth. It completely ignores possible “cryptic” female choice that steers fertilization and the fate of fertilized eggs.
It was once widely presumed that mating reliably indicates paternity. Indeed, alpha males generally copulate most and often monopolize females at peak fertility, so it seems obvious that they sire most infants. But from the late 1980s reliable paternity testing increasingly revealed exceptions.
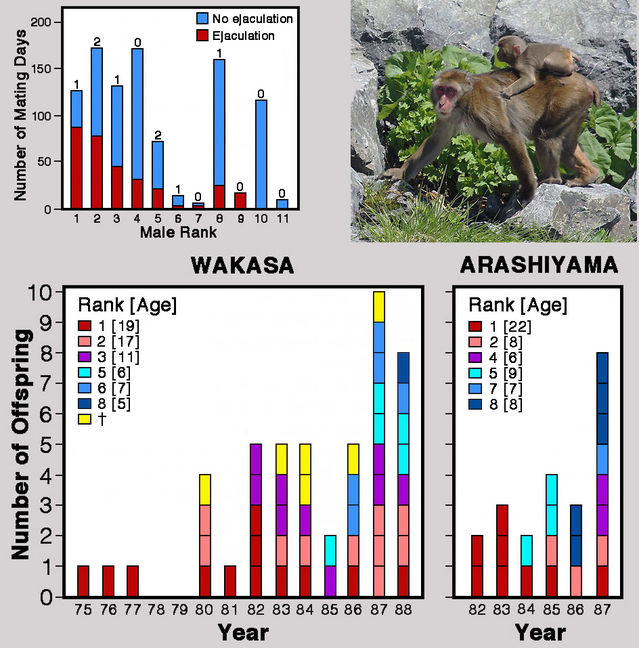
A striking example of the complexities of primate mating relationships was provided in a 1991 report on captive Japanese macaques by Miho Inoue and colleagues. Like other authors, they found that copulation frequency was positively associated with male rank, especially when ejaculation occurred. But the newly developed method of DNA fingerprinting revealed that paternity was not related to mating frequency. Even low-ranking males, which rarely copulated, fathered infants. Yet an even greater surprise was in store: During the mating season, Inoue and colleagues observed continuously throughout every day, recording every single copulation. The third- and sixth-ranking males each sired one infant but were never seen to copulate with the mothers! Their successful matings must have happened by night.
A different factor in mate choice
Since 1992, many studies of primates and other animals have shown that mating frequency does not consistently indicate paternity. Reliable conclusions require genetic tests. A radical alternative to the notion that dominant males have “good genes” is in fact connected with the immune system.
A well-developed capacity for fighting infections is crucial for survival, but invading microbes have a major advantage over any large-bodied host: Microbes breed much faster and respond far more rapidly to natural selection, so they swiftly develop adaptations to evade the host’s defenses. In response, early animals with backbones (vertebrates) developed acquired immunity. The surfaces of almost all cells present fragments of foreign proteins (antigens) bound to special molecules produced by genes of the Major Histocompatibility Complex (MHC). This is an unusually large gene family (numbering over 200 in humans), mostly with multiple alternative versions permitting millions of unique combinations. Production of many different MHC molecules by an infected cell increases the odds that one or more will bind to a foreign protein fragment and display it, triggering an immune response from special white blood cells.
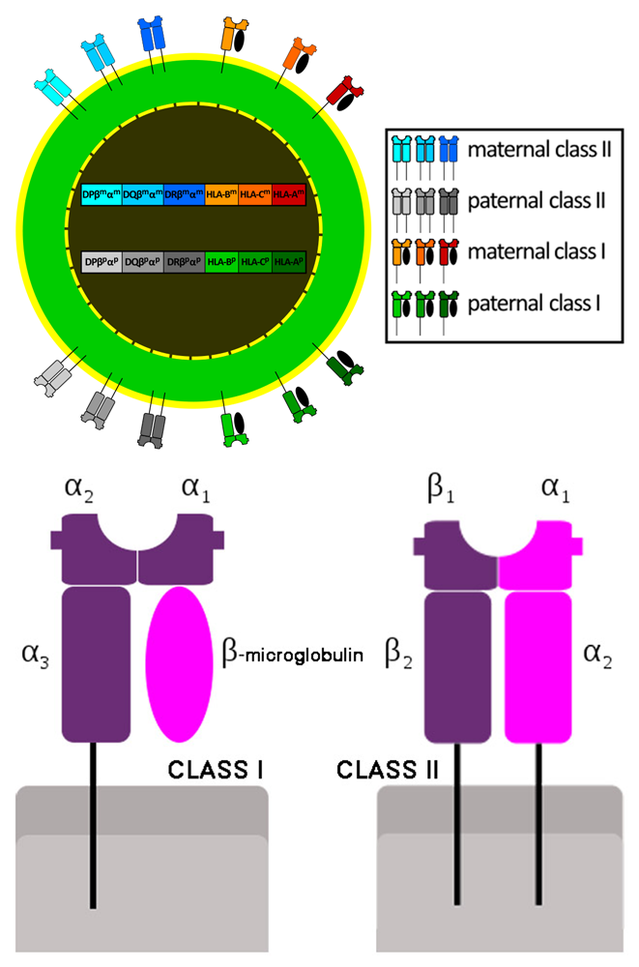
Immunity and mate choice are connected because natural selection should optimize the offspring’s array of MHC genes. It makes sense for both sexes to seek partners with different MHC genes so that arrays in offspring will be sufficiently variable to combat infections successfully. The goal is not to find a mate with “good genes” but one with “compatible genes”. So a dominant male in a social group may not be a suitable mate for all females present.
MHC genes and mate choice
In a nutshell, an individual can increase offspring survival by choosing a mate with optimally complementary MHC genes. This idea is not new, but it has taken many years to investigate relationships between MHC and mate choice across all jawed vertebrates — fish, amphibians, reptiles, birds, and mammals. Over 20 different species (including sticklebacks) have now been studied, with broadly similar results.
For mammals, the first good evidence that mate preferences are linked to MHC genes was reported for mice. In 1976, Kunio Yamazaki and others published a now classic paper on tests in which male mice that chose between two receptive females predominantly preferred one with dissimilar MHC genes. In the meantime, many experiments conducted with mice have confirmed and expanded the initial findings. It is now known that foreign protein fragments bound to portions of MHC molecules are detected by the vomeronasal apparatus — an accessory olfactory system specifically adapted to detect odor signals within a species.
Several studies have been conducted on the relationship between MHC genes and mating behavior in primates, confirming that individuals mostly prefer mates with dissimilar MHC genes. Recent work has been conducted with free-living mouse lemurs — small, relatively primitive primates that combine solitary nocturnal habits with promiscuous mating. In 2008, Nina Schwensow and colleagues reported that genetically identified fathers differed more from mothers in MHC types than other males. In fact, they found that some kind of cryptic female choice operates after copulation. A subsequent study of mouse lemurs reported by Elise Huchard and colleagues in 2013 similarly identified MHC‐dependent mating combinations. However, they also found evidence for inbreeding avoidance, indicating a dual effect.
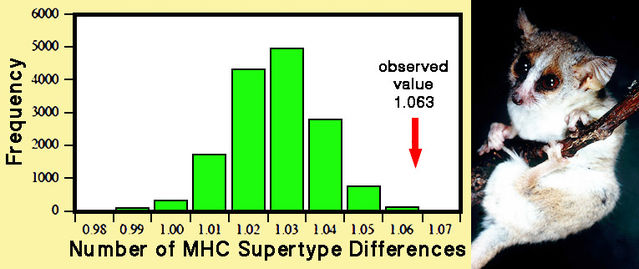
Considerably more research has been conducted on higher primates, notably macaques, baboons, and mandrills. A 2010 paper by Joanna Setchell and colleagues examined reproduction in a population of mandrills (mentioned above), ranging within a large enclosure. For almost 200 infants, genetic characteristics of the father — compared with all other possible sires — and degree of genetic difference from the mother were assessed. The probability of any male siring an infant increased as his relatedness to the mother decreased, while the degree of dissimilarity from the mother in MHC genes and overall genetic profile increased. These effects were detected despite the fact that social rank strong influences male reproductive success.
Humans have it too
Turning to humans, a landmark 1992 paper by Carole Ober and colleagues reported on relationships between reproductive outcome and the MHC system in Hutterites — a reproductively isolated North American community descended from European ancestors. This team had previously reported that couples with a similar MHC type had longer intervals between marriage and first birth. They later examined MHC similarity in relation to fertilization success and fetal loss. Couples sharing a particular MHC similarity were found to have significantly higher rates of fetal loss than other couples. A follow-up 1997 paper, analyzing information from over 400 Hutterite couples, revealed that spouses showed significantly fewer MHC matches than expected by chance.
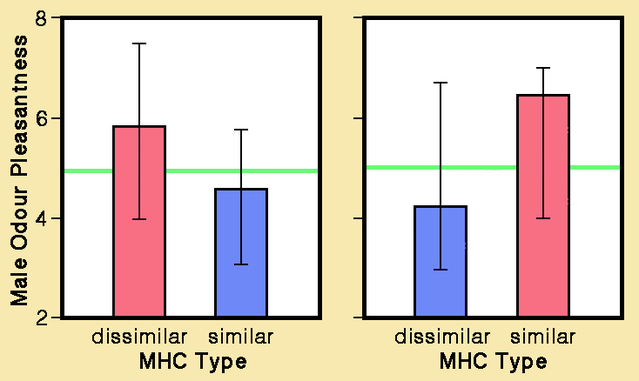
In now-famous experiments reported by Claus Wedekind and colleagues in 1995, students rated odors of T-shirts worn for two nights by members of the opposite sex. Naturally cycling female testers scored male body odors as more pleasant if their MHC types were distinctly different. Moreover, odors of MHC-dissimilar men reminded women testers more often of actual or former partners. Unexpectedly, however, the difference in odor rating was reversed in women using oral contraceptives, who preferred odors of men with similar MHC types. In 1997, Wedekind and Sandra Füri reported that partner preferences based on odor differences seemingly enhanced general genetic variability in offspring rather than favoring specific MHC combinations.
It’s complicated
Humans share the general vertebrate pattern of preferences that tend to increase the diversity of MHC genes in offspring. It is even possible that choice favors specific beneficial gene combinations. However, mate preferences may also avoid inbreeding by enhancing general genetic variability. Note, however, that — in addition to avoiding inbreeding depression when offspring have too many similar genes — it is also important to avoid outbreeding depression, when parental genes are too different. Accordingly, various studies have indicated that mate choice tends to promote a moderate degree of difference in MHC genes. Finally, although the simplistic notion of one-size-fits-all “good genes” conflicts with mechanisms involving “compatible genes”, we must remember that under certain conditions social rank does definitely influence mating success as well.
References
Boehm, T. & Zufall, F. (2006) MHC peptides and the sensory evaluation of genotype. Trends in Neurosciences 29:100-107.
Dixson, A.F. (2016) The Mandrill: A Case of Extreme Sexual Selection. Cambridge, UK: Cambridge University Press.
Huchard, E., Baniel, A., Schliehe-Diecks, S. & Kappeler, P.M. (2013) MHC-disassortative mate choice and inbreeding avoidance in a solitary primate. Molecular Ecology 22:4071-4086.
Inoue, M., Mitsunaga, F., Ohsawa, H., Takenaka, A., Sugiyama, Y., Gaspard, S.A. & Takenaka, O. (1991) Male mating behaviour and paternity discrimination by DNA fingerprinting in a Japanese macaque group. Folia Primatologica 56:202-210.
Inoue, M., Mitsunaga, F., Ohsawa, H., Takenaka, A., Sugiyama, Y., Soumah, A.G. & Takenaka, O. (1992) Paternity testing in captive Japanese macaques (Macaca fuscata) using DNA fingerprinting. pp. 131-140 in: Paternity in Primates: Genetic Tests and Theories. Implications of Human DNA Fingerprinting. (eds. Martin, R.D., Dixson, A.F. & Wickings, E.J.), Basel: Karger.
Knapp, L.A. (2005) The ABCs of MHC. Evolutionary Anthropology 14:28-37.
Leinders-Zufall, T., Brennan, P., Widmayer, P., Chandramani, P., Maul-Pavicic, A., Jäger, M., Li, X.-H., Breer, H., Zufall,F. & Boehm, T. (2004) MHC class I peptides as chemosensory signals in the vomeronasal organ. Science 306:1033-1037.
Ober, C., Elias, S., Kostyu, D.D. & Hauck, W.W. (1992) Decreased fecundability in Hutterite couples sharing HLA-DR. American Journal of Human Genetics 50:6-14.
Ober, C., Weitkamp, L.R., Cox. N., Dytch, H., Kostyu, D. & Elias, S. (1997) HLA and mate choice in humans. American Journal of Human Genetics 61:497-504.
Schwensow, N., Eberle, M. & Sommer, S. (2008) Compatibility counts: MHC-associated mate choice in a wild promiscuous primate. Proceedings of the Royal Society of London B 275:555-564.
Setchell, J.M., Charpentier, M.J.E., Abbott, K.M., Wickings, E.J. & Knapp, L.A. (2010) Opposites attract: MHC-associated mate choice in a polygynous primate. Journal of Evolutionary Biology 23:136-148.
Wedekind, C. & Füri, S. (1997) Body odour preferences in men and women: Do they aim for specific MHC-combinations or simply heterozygosity? Proc. Roy. Soc. Lond. B 264:1471-1479.
Wedekind, C., Seebeck, T., Bettens, F. & Paepke, A.J. (1995) MHC-dependent mate preferences in humans. Proceedings of the Royal Society of London B 260:245-249.
Yamazaki, K., Boyse, E.A., Miké, V., Thaler, H.T., Mathieson, B.J., Abbott, J., Boyse, J., Zayas, Z.A. & Thomas, L. (1976) Control of mating preferences in mice by genes in the major histocompatibility complex. Journal of Experimental Medicine 144:1324-1335.




