
While our recent look at residential solar may lead you to believe harnessing that power is a newer initiative, humans have been exploiting solar energy for thousands of years to heat their homes, cook, and produce hot water. Some of the earliest written references to technology consciously designed to capture the Sun’s rays come from ancient Greece. Socrates himself said, “in houses that look toward the south, the sun penetrates the portico in winter, while in summer the path of the sun is right over our heads and above the roof, so that there is shade.” He is describing how Greek architecture exploited the different paths of the Sun through the sky at different times of the year.
By the fifth century BCE, the Greeks were struggling with an energy crisis. Their predominant fuel, charcoal from trees, was scarce since they had stripped their forests in order to cook and heat their houses. Wood and charcoal were rationed, and olive groves needed protection from the citizenry. The Greeks addressed their energy shortage by carefully planning the layout of their cities to ensure that each house could take advantage of the sunshine in the way Socrates described. The combination of technology and enlightened government policy worked, and a crisis was avoided.Technologies for harnessing the thermal energy in sunlight have only continued to grow over time. Colonists in New England borrowed the ancient Greek homebuilding techniques to keep warm in the harsh winters. Simple passive solar water heaters, little more than a black-painted barrel, were sold commercially in the United States in the late 19th century. And more elaborate solar heating systems were developed to pipe water through absorbing and/or focusing panels. The hot water is stored in an insulated tank until needed. In climates subject to freezing, a two-fluid system is used, where the Sun heats a water/antifreeze mixture that passes through coils embedded in the storage tank, which does double-duty as a heat exchanger.
These days, a variety of sophisticated commercial systems are available for water and space heating in the home. Solar thermal systems are deployed throughout the world, with the largest installed base per capita found in Austria, Cyprus, and Israel.

But modern solar truly starts in 1954 with the discovery of a practical way to make electricity from light: Bell Labs uncovered the fact that silicon could make a photovoltaic material. This finding created the foundation for today's solar cells (essentially the devices converting light energy into electricity) and ushered in a new era of solar power. Aided by intense research ever since, it's an era that continues today as solar appears poised to become the dominant source of power in the future.
What is a solar cell?
The most common type of solar cell is a semiconductor device made from silicon—a cousin of the solid-state diode. The familiar solar panels are made from a number of solar cells wired together to create the desired output voltage and current. Those cells are surrounded by a protective package and topped with a glass window.
Solar cells generate electrical power using the photovoltaic effect, a fact that didn't come from Bell Labs. Instead, this was first discovered in 1839 by French physicist Alexandre-Edmond Becquerel (son of physicist Antoine Cesar Becquerel and father of physics Nobelist Henri Becquerel, the discoverer of radioactivity). A little more than a century later, Bell Labs had its solar cell breakthrough, providing the foundation of the most common solar cells.
In the language of solid state physics, a solar cell is formed from a p-n junction in a silicon crystal. The junction is made by “doping” different areas of the crystal with small amounts of different impurities; the interface between these regions is the junction. The n side is a conductor with electrons as the carriers of current, and the p side has “holes,” or areas with missing electrons that act as current carriers within the crystal. In the region near the interface, the diffusion of charges creates a local “built-in voltage” across the interface. When a photon enters the crystal, if it has enough energy, it may dislodge an electron from an atom, creating a new electron-hole pair.
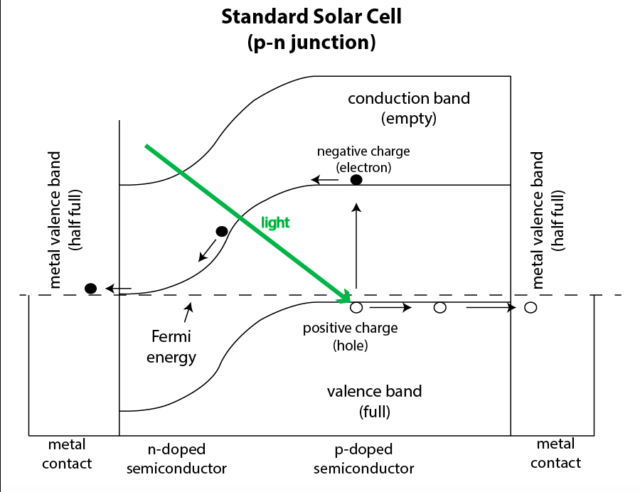
The newly freed electrons are attracted to the holes on the other side of the junction, but they are prevented from crossing it due to the built-in voltage. However, if a pathway is provided through an external circuit, the electrons can travel through it and light our homes along the way. When they reach the other side, they recombine with the holes. This process can continue as long as the Sun continues to shine.
The energy required to transform a bound electron into a free one is called the “band gap.” It’s the key to understanding why photovoltaic (PV) cells have an intrinsic limit on efficiency. The band gap is a fixed property of the crystal material and its dopants. Those dopants are adjusted so that solar cells have a band gap close to the energy of a photon in the visible region of the spectrum. This is a practical choice, because visible light isn’t absorbed by the atmosphere (phrased differently, we humans have evolved to see in the most common wavelengths).
Photons come in fixed amounts of energy, which means their energy is quantized. That also means a photon with energy less than the band gap (say, one in the infrared part of the spectrum) won’t create a charge carrier. It will simply heat the panel. Two infrared photons together will do no better, even if their combined energy would be enough to bridge the gap. A photon with excess energy (an ultraviolet photon, for example) will knock an electron loose, but the excess energy will also be wasted.
Since efficiency is defined as the ratio of light energy striking the panel divided by electrical energy extracted—and since much of this light energy will necessarily be wasted—the efficiency can not be 100 percent.
The band gap of a silicon PV solar cell is 1.1 electron volts (eV). As can be seen from the diagram of the electromagnetic spectrum reproduced here, the visible spectrum lies just above this, so visible light of any color will produce electrical power. But this also means that for each photon absorbed, excess energy is wasted and converted into heat.
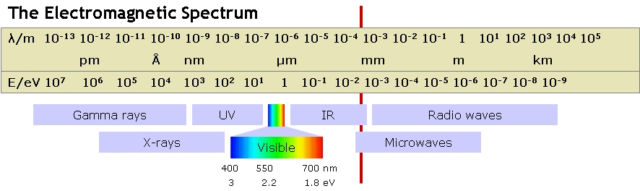
The upshot is that even if the PV panel is flawlessly manufactured and conditions are ideal, the theoretical maximum efficiency is about 33 percent. Commercially available solar panels typically achieve about 20 percent efficiency.
Perovskites
Most of the solar panels commercially deployed are made from the silicon cells described above. But research into other materials and strategies is underway in laboratories around the world.
Some of the most promising recent research for silicon alternatives has involved materials called perovskites. The mineral perovskite (CaTiO3) was named in 1839 in honor of Count Lev Aleksevich Perovski (1792-1856), a Russian mineralogist. It can be found on every continent and in the clouds of at least one exoplanet. The word “perovskite” is also used for synthetic compounds that have the same orthorhombic crystal structure as the naturally occurring mineral (or a closely related one) and share a structurally similar chemical formula.
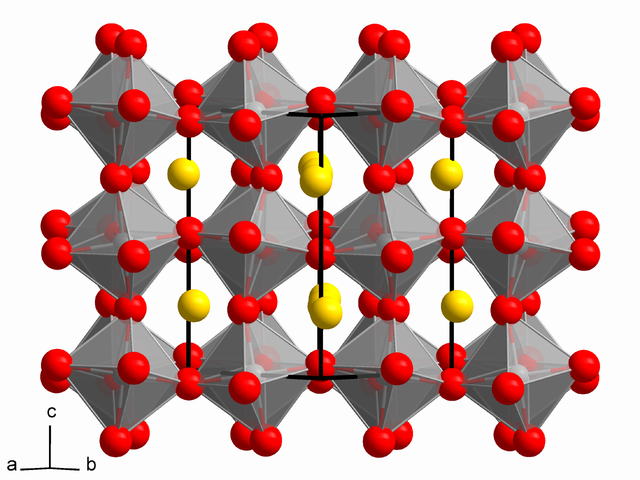
Depending on which elements are used, perovskites can display a wide variety of useful properties, such as superconductivity, giant magnetoresistance, and photovoltaic activity. Their use in PV cells has generated a great deal of optimism, as they have shown an unprecedented increase in efficiency from 3.8 percent to 20.1 percent in the past seven years of laboratory research. This rapid rate of progress inspires confidence that further gains are likely, especially as the factors limiting efficiency are becoming clearer.
reader comments
198