Vitamins and the Failure of Free-Market Health
The booming dietary-supplement industry is plagued by outlandish claims, undermining credible science, and seeding confusion.

A few days ago I found a browser tab open, and I don’t know how it got there. It was a journalistic-looking page that has since been taken down. At the top was what appeared to be a screenshot of CNN anchor Anderson Cooper interviewing astrophysicist Stephen Hawking, overlaid with “BREAKING NEWS,” and a quote presumably attributed to Hawking “We can now access 100 percent of the brain.”
The article that followed was dated April 6, 2017. It began with a shocking revelation: “Stephen Hawking credits his ability to function and maintain focus on such a high level to a certain set of ‘smart drugs’ that enhance cognitive brain function and neural connectivity while strengthening the prefrontal cortex and boosting memory recall.” The URL was socialaffluent.com.
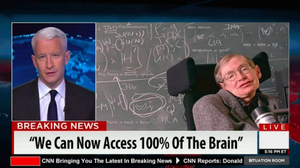
“In an interview with Anderson Cooper, Stephen Hawking said that his brain is sharper than ever, more clear and focused, and he credits a large part to using Synagen IQ,” it read. “Hawking went on to add, ‘The brain is like a muscle, you got to work it out and use supplements just like body builders use, but for your brain, and that’s exactly what I’ve been doing to enhance my mental capabilities.’”
Hawking, of course, did not say this to Cooper. That’s evident in the bizarre syntax and the fact that Hawking is not one for redundant clauses (“but for your brain”). I even found a blog with the same text that substituted Wolf Blitzer for Anderson Cooper. The whole thing is fiction. Except for the product itself.

You can currently buy Synagen IQ on Amazon at $67.76 for 30 pills of the “natural brain enhancer” that “supports cognitive performance.” Amazon may preclude fictional endorsements from celebrity scientists, but it does allow the seller, Phenom Health, to say that the product will “drastically improve every aspect of your brain.”
With humanity teetering on the brink of an epidemic of longevity-related dementia, even a pitch this absurd can win customers. Well-read people could come to reason that a product with even a 1-percent chance of doing anything to help is worth a shot. The health marketplace does not invite rational behavior, even when information is perfect.
While pills like Synagen IQ appear to be marketed at the darkest end of the disinformation spectrum, the category of products sold as “dietary supplements” is vast and corybantic. It also includes compounds for which valid cases exist, like prenatal folate and vitamin D pills. In all, this umbrella distinction has grown into an industry that claims to contribute $122 billion and 754,645 jobs to the U.S. economy each year.
The future of products like this hangs in the balance as Donald Trump’s nominee to lead the Food and Drug Administration (FDA), Scott Gottlieb, now sits for confirmation hearings. At stake is more than jobs and money—what can legally be implied or promised by sellers, when products can come to market, when they must be removed—but a groundwork for fundamental trust in medical therapeutics and scientific expertise.
On March 22, the world’s leading supplement advocacy group, the Natural Products Association, marked its 20th Annual Natural Products Day by canvassing Capitol Hill to lobby for supplements. The group is led not by a band of Birkenstock-wearing spirit healers, but mostly men in business suits. As the supplement lobbyists put it in a statement, their mission was to “educate members of Congress and legislative staff about the important role natural products play in keeping Americans healthy.”
The “natural products” in question are not fruits, vegetables, nuts, seeds, or even legumes. They are compounds like those traditionally known as vitamins––in concentrations and formulations that may or may not occur in nature––as well as enzymes, pre-hormones, various metabolites and glandulars, etc. The supplement industry operates in a rogue position relative to the pharmaceutical industry. While both sell body-modifying chemicals, supplements—which the FDA regards more like foods than drugs—can go straight to market without providing evidence of efficacy or purity.
The likely new commandant Gottlieb has made news over the years for his libertarian leanings and ties to private industries, namely pharmaceutical. He has accused the FDA of “evading the law.” A former editor of The New England Journal of Medicine said Gottlieb has “an orientation which belies the goal of the FDA,” and last month the journal published a scathing note of censure:
Our health system depends on the shared belief that drugs and devices work as advertised, that mechanisms invisible to the eye nonetheless cause healing. The underpinnings of this belief are that “somebody out there” has tested these products and shown, with at least some scientific evidence, that they work as claimed.
The “somebody out there” is the FDA, a small office of which oversees all supplement products, though its capacity is limited to conducting occasional audits and acting on some consumer complaints. The FDA defines supplements circularly: “A dietary supplement is a product taken by mouth that is intended to supplement the diet and that contains one or more ‘dietary ingredients.’” These dietary ingredients “may include vitamins, minerals, herbs or other botanicals, amino acids, or other substances found in the human diet, such as enzymes.”
This nebulous definition that is not likely to narrow under Gottlieb, according to supplement trade organizations that have expressed support for his nomination to lead the administration. Steve Mister of the Council for Responsible Nutrition has said, “I think he shares a lot of the same concerns we have about overregulation.”
The CEO of the Natural Products Association, Dan Fabricant, is also optimistic. His goals for the industry go well beyond avoiding overregulation. Some were part of the March lobbying day.
“Our talking points on the Hill were with regard to expanding health savings accounts to include dietary supplements,” Fabricant told me. “Our concerns are also for underserved communities,” he continued, referring to limitations with regard to using food stamps to buy supplements. “We’re looking to add multivitamins as a choice to folks who’ve fallen on hard times.”
Those scenarios together would make supplements purchasable using health-care money as well as food money, and that gets to the heart of the matter. Which are they, food or medicine? What are supplements trying to be? And then, also important, what are they, really?

When I last looked at Fabricant’s portrait on the Natural Products Association’s website, there was an adjacent ad for a product called Carditone that promised “all natural blood-pressure support.”
That wording is key. Because supplements are legally distinct from pharmaceuticals, purveyors can’t make claims about curing, treating, preventing, or mitigating a disease (“other than a classical nutrient deficiency disease,” like scurvy or beriberi). But why buy a pill that isn’t curing, treating, preventing, or mitigating a disease? Enter artful ways of offering things like “natural brain enhancer” or “all natural blood pressure support,” which implies much but is beyond provoking regulatory rebuke or litigious saber rattling should a user die of a brain hemorrhage.
Even though the supplement industry is adamantly not selling medicine, medicalized tones are pervasive in the marketing. For example, Fabricant is pitched by his publicists as, in full, “Dr. Daniel Fabricant,” which is also how he identifies on Twitter. The nature of his honorific is elucidated on the Natural Products Association’s site, where he is “Dr. Daniel Fabricant, Ph.D.” His Ph.D. is in pharmacognosy, a field defined by his alma mater as “the chemistry, biochemistry, biology, taxonomy, and ethnobiology of natural products.”
I asked about looming budget cuts to the FDA, on the order of $40 million in President Trump’s proposed plan. Fabricant said we have no idea if the FDA is actually going to get cut. And even if it does, “good regulators find a way to get things done even in the face of cuts.”
Fabricant himself came to the supplement industry by way of the FDA, where he was director of the division of dietary supplements (before it became a full-fledged office). Based on that experience, he said, he’s also advocating for a clearer definition of something called medical food, which is another vague category of products—a potentially hot market currently defined by a few nebulous lines in 1983’s orphan drug act.
I reacted by asking him about broccoli and salads, and why those aren’t medical foods. They make people with diabetes and heart disease less sick, when used regularly. Conversely, chronic abuse of Pop-Tarts and Pepsi contributes to lethal disease. Eating mostly whole plants will protect most hearts more effectively than the most widely prescribed cardio-protective pharmaceuticals, statins, and yet food is not medication.
“Well, no, that’s not what we’re talking about. We’re talking about something people don't get in their diet, or they don’t get enough,” Fabricant said. “I imagine that if people ate concentrated forms of broccoli for recovery or something like that, they may have a benefit as well. So it’s a matter of concentration.”
So if it occurs in at least some form in the diet, then it’s your domain? And if it’s something that never occurs in any food anywhere, then it’s a pharmaceutical?
“More than likely. I mean, the whole thing is the intent to cure, treat, or mitigate a disease. I don’t think what Frank and I are talking about is curing, treating, or mitigating a disease.”
Here I should mention there was also a man named Frank Jaksch on my call with Fabricant. I wasn’t fully expecting that. He’s the head of a company called Chromadex that describes itself as “an innovator of proprietary health, wellness, and nutritional ingredients.” It is not a pharmaceutical company, though its work is somewhere at the opposite end of the supplement spectrum from the purveyors of Synagen IQ. Jaksch twice mentioned to me that he’s developing a “novel vitamin” called nicotinamide riboside, and that there’s science behind the compound, including a study published in Nature Communications last October. Chromadex’s press release at the time said, “Published studies in humans and mice reveal how a superior vitamin B3 may play an important role in helping us enjoy longer, healthier lives.”
Note that this is not a claim to cure, prevent, treat, or mitigate a disease. So where does the length and health come in?
“I mean, maybe it’s post-operative,” said Fabricant. “Maybe people recover faster from a surgery if they have added nicotinamide riboside. Maybe it slows the rate of progression of diseases. That’s not necessarily—it may be ‘treating,’ but I think that those are the, you know, the discussions that we think the agency should be having.”
Jaksch frames nicotinamide riboside as a nutrient in which we are all deficient, and that framing makes it a supplement, or vitamin, as he prefers. He also said that the medical foods path to market “would be a good one to go after.”
Nicotinamide riboside is rare among supplement compounds in that it is being studied extensively, in trials funded by Chromadex and other parties. According to clinicaltrials.gov, 12 clinical trials involving nicotinamide riboside are ongoing or completed, many of which sound a lot like those done for pharmaceuticals. At the University of Washington, one is assessing “the pharmacokinetics of NR at a maximum dose of 1,000 milligrams as well as the safety and tolerability of NR.” At the University of Colorado, Boulder, a trial is assessing the efficacy of the compound “for improving physiological function (vascular, motor, and cognitive) in healthy middle-aged and older adults.”
Fabricant points to the work of Chromadex as exemplary in taking a research-driven approach. Though all of this seems to blur the line further between a supplement, or part of a medical food, and a drug. Why wouldn’t nicotinamide riboside be a pharmaceutical? Because it’s found somewhere in nature, somewhere in our food supply?
“It’s a milk metabolite, so it’s found in cow’s milk in some quantity,” said Jaksch. “But it’s there in small amounts. An average adult is not going to be able to drink enough milk to get enough nicotinamide riboside to cover their dietary needs.”
A metabolite that no one could ever get from food, and in which nearly 100 percent of people are deficient, represents a big market. And while consumers are waiting for clinical trials to play out, people don’t need to wait to buy nicotinamide riboside. You might have seen the ads on Facebook for Elysium, a company selling nicotinamide riboside as a supplement called Basis, “the one daily supplement your cells need” which is “designed to support well-being at the cellular level.”
Since I’m still unclear on why this compound isn’t a pharmaceutical, and why it shouldn’t be tested for efficacy and safety in many thousands of people before it goes to market, Fabricant backs up and scopes out: “We take a few things as kind of the simple razors here. We know that food can play a role in disease—whether that’s quicker healing, a diminished rate of developing the disease, slowing a degenerative disease like Parkinson’s or Alzheimer’s. I think we’ve seen the evidence from science that there are nutrients out there that do that exact thing. So if we take that to be the understanding, there’s not a clear regulatory structure to get those products in the hands of consumers. What’s the burden of evidence that’s required to make those sorts of claims? It’s a complete nebulous area that nobody, including the FDA, has a clear answer for.”
That seems to be the nut of it, a genuine desire from all parties for a clear process that lets consumers know what they’re getting but isn’t a burden to corporate purveyors and, as President Trump so often emphasizes, jobs. But what if you are trying to create a path to market for a thing that already has a path to market, either as a food or as a pharmaceutical?
The free-market approach to supplements fails because of the subtleties and timescales of human health: The reasoning that bad products will simply fail in the free marketplace falls apart when people are unable to tell if a product is bad. If a certain model of car kept making the nightly news because it had a propensity for breaking into flames or getting only half of its purported gas mileage, consumers would presumably avoid that car. The invisible hand would clap for itself.
The situation is different for gurus and corporations selling body optimization. There is no equivalent way for a consumer to know if a cardiac serum is truly supporting one’s heart or if a brain pill is indeed helping to stay a diminishment of capacities. (“Like body builders use, but for your brain.”) A market flooded with such products distracts from legitimate therapeutics, turns people against expertise, stokes tendencies toward shortcuts to health, wastes money and hope, perverts the idea of nature, and distracts from the imperative of prioritizing quality food as though it were medicine.