Food and Drug Administration reports obtained by STAT through a Freedom of Information Act request detail the heart-rending stories of babies and toddlers who became severely ill or died after taking homeopathic teething products—which, as Ars has reported, the FDA has found to contain inconsistent amounts of toxic belladonna, aka deadly nightshade.
The reports describe more than 370 infants becoming ill, with symptoms including twitching, seizing, losing consciousness, and turning blue. The illnesses have required emergency treatment and in some cases babies being airlifted to hospitals. Many of the symptoms are consistent with belladonna poisoning, which is known to cause seizures, vomiting, difficulty breathing, lethargy, excessive sleepiness, muscle weakness, skin flushing, constipation, difficulty urinating, blurred vision, and confusion.
In response to warnings last year from the FDA, Hyland’s stopped distributing the products in the US. However, Hyland’s has continued to insist that the products are safe and has refused to recall them. They can still be found in some retailers and online.
“My daughter had a seizure, lost consciousness, and stopped breathing about 30 minutes after I gave her three Hyland’s Teething Tablets,” one FDA report read. “She had to receive mouth-to-mouth CPR to resume breathing and was brought to the hospital.”
Another report included a handwritten note from a physician to Hyland’s stating that his five-month-old patient was unresponsive for 45 minutes after taking the teething tablets. “I would like you to report it, find a contact at the FDA, so we can start an investigation and pull this dangerous, unregulated product from the shelves,” the doctor wrote.
One mother described how her child’s pupil’s dilated “like marbles.” Another reported that her daughter continued to have seizures after taking the tables. “I hate hate hate u for this,” she wrote to Hyland’s.
And then there were the death reports. A grandmother recounted how her five-month-old grandson died in his sleep after taking the tablets. When he was found, his body was still 102 degrees Fahrenheit. The cause of death was ruled as acute cardiopulmonary arrest. “SOMEONE NEEDS TO INVESTIGATE THIS!!!!” she wrote.
In a 2014 incident, a nine-month-old girl died within 45 minutes of taking tablets for the first time. She was found in her crib alongside a splatter of vomit. Her mother contacted Hyland’s months later after hearing that the tablets could cause seizures. But by then, she had thrown out her bottle of teething tablets and the company declared that no further investigation was possible.
The case highlights how difficult it is to track down the source of such poisonings—and in part why it may have taken so long for the FDA to issue warnings, which it did in 2010 and 2016.
The FDA does not review or approve homeopathic medicines and has little power to enforce a recall. However, the fact is muddled by homeopathic manufacturers, who often offer false and baseless assurances that their products are safe, effective, and rigorously regulated.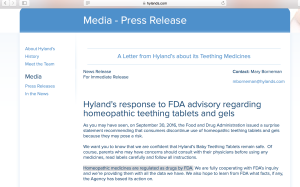
For instance, in an undated press release from last year, Hyland’s stated, “Homeopathic medicines are regulated as drugs by FDA.” They are not. In fact, Hyland's homepage now has a tiny disclaimer that states, “The uses for our products are based on traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.”
As STAT reported, Connecticut Democratic Rep. Rosa DeLauro, introduced a bill last week, called the Recall Unsafe Drugs Act, which would give the FDA mandatory recall authority over homeopathic products and drugs.
In a news release, DeLauro was quoted as saying:
Hyland’s refusal to recall its teething tablets, despite numerous health and safety warnings from the FDA, is downright shameful. While the FDA has called on consumers to stop using the products, Hyland’s will not go above and beyond what the law requires and is choosing instead to prioritize the company’s profits and reputation before the safety of children… There is absolutely no reason why a potential life-threatening product should be in our families’ homes where unsuspecting parents may give it to their children.
reader comments
297