Graphene: The New Superman of Materials
Graphene had an exceptional year in 2016. The material became a superconductor. It strengthened new innovations. It even started to change color. However, graphene has one more surprise in store. A new study proved that graphene’s electrons react faster than previously thought. Thus, graphene can handle far more electrical current than other materials, solidifying its […]

Graphene had an exceptional year in 2016. The material became a superconductor. It strengthened new innovations. It even started to change color. However, graphene has one more surprise in store.
A new study proved that graphene’s electrons react faster than previously thought. Thus, graphene can handle far more electrical current than other materials, solidifying its place as the go-to electronic building block.
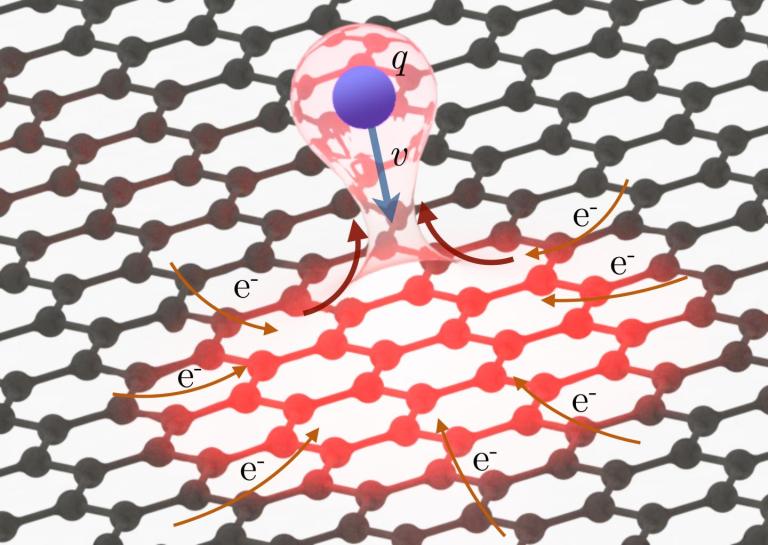
A team from Austria’s Institute of Applied Physics at TU Wien showed just how quick the electrons in graphene can be. The impact of xenon ions with a highly-charged graphene film caused electrons to be stripped from a spot. Each xenon atom can remove 20 electrons in any one area. Given that each carbon atom only has six electrons, the xenon could’ve easily ruined graphene’s stability.
 [Image Source: Gruber et al, Eurekalert]
[Image Source: Gruber et al, Eurekalert]
“The current density is around 1,000 times higher than that which would lead to the destruction of the material under normal circumstances,” said one of the researchers, Elisabeth Gruber,
What shocked researchers was the material’s ability to replace the electrons in those spots in just femtoseconds (one quadrillionth of a second).
In any other material, the electrons would move to patch the gaps and restore stability. However, it wouldn’t happen fast enough and the material’s structure would effectively be compromised. Team member Richard Wilhelm from the Helmholtz-Centre Dresden-Rossendorf in Germany explained it as such:
“What you would expect to happen now is for these positively charged carbon ions to repel one another, flying off in what is called a Coulomb explosion and leaving a large gap in the material. But astoundingly, that is not the case. The positive charge in the graphene is neutralized almost instantaneously.”
Graphene’s structure is comprised of one-atom-thick carbon in a lattice network. On a nano scale, it displays some incredible properties. Graphene can serve as a superconductor. It’s incredibly strong and hard, yet flexible for production. However, no one has yet to pinpoint exactly where graphene gets these traits.
 [Image Source: ZMeScience]
[Image Source: ZMeScience]
Despite not knowing all of the substance’s mysteries, the researchers hope to utilize these properties in highly efficient electronics.
“The hope is that for this very reason, it will be possible to use graphene to build ultra-fast electronics. Graphene also appears to be excellently suited for use in optics, for example in connecting optical and electronic components,” said lead researcher Professor Fritz Aumayr.
 SHOW COMMENT ()
SHOW COMMENT ()









